
The $H-O-H$ angle in water molecule is about:
A) ${{90}^{\circ }}$
B) ${{180}^{\circ }}$
C) ${{102}^{\circ }}$
D) ${{105}^{\circ }}$
Answer
566.7k+ views
Hint: This is based on the basic concept of VSEPR theory which explains that due to the lone pair- lone pair repulsion, its geometry is not tetrahedral but distorted to bent V-shaped shaped and also there is slight change in the bond angle of tetrahedral also. Now answer the statement.
Complete Solution :
- We can know the bond angle in water molecules through the VSEPR. VSEPR stands for the valence shell electron pair repulsion theory or simply we can say that VSEPR theory. This theory gives us information about the bond angles, bond length etc.
Now considering the statement:
In a water molecule, we know that there are two hydrogen atoms which are attached to the one oxygen atom with the oxygen atom being the central atom.
- The electronic configuration of the oxygen atom in the ground state is as 2, 6 i.e. it has 6 electrons in its outermost valence.
- The two hydrogen atoms contribute one electron each and therefore, the oxygen is left with a total of four electrons which are present in the form of two lone pairs.
- Therefore, for the coordination number 4 that is total of eight electrons or 4 electron pairs the hybridization of the water molecule is $s{{p}^{3}}$ and the geometry for this hybridization is tetrahedral and its bond angle should be ${{109}^{\circ }}{{5}^{'}}$ but due to lone pair repulsion, its bond angle reduces to ${{104}^{0}}5'$. So, thus, water has bent V-shaped structure and therefore is considered to have distorted tetrahedral geometry.
The structure of water molecule is as:
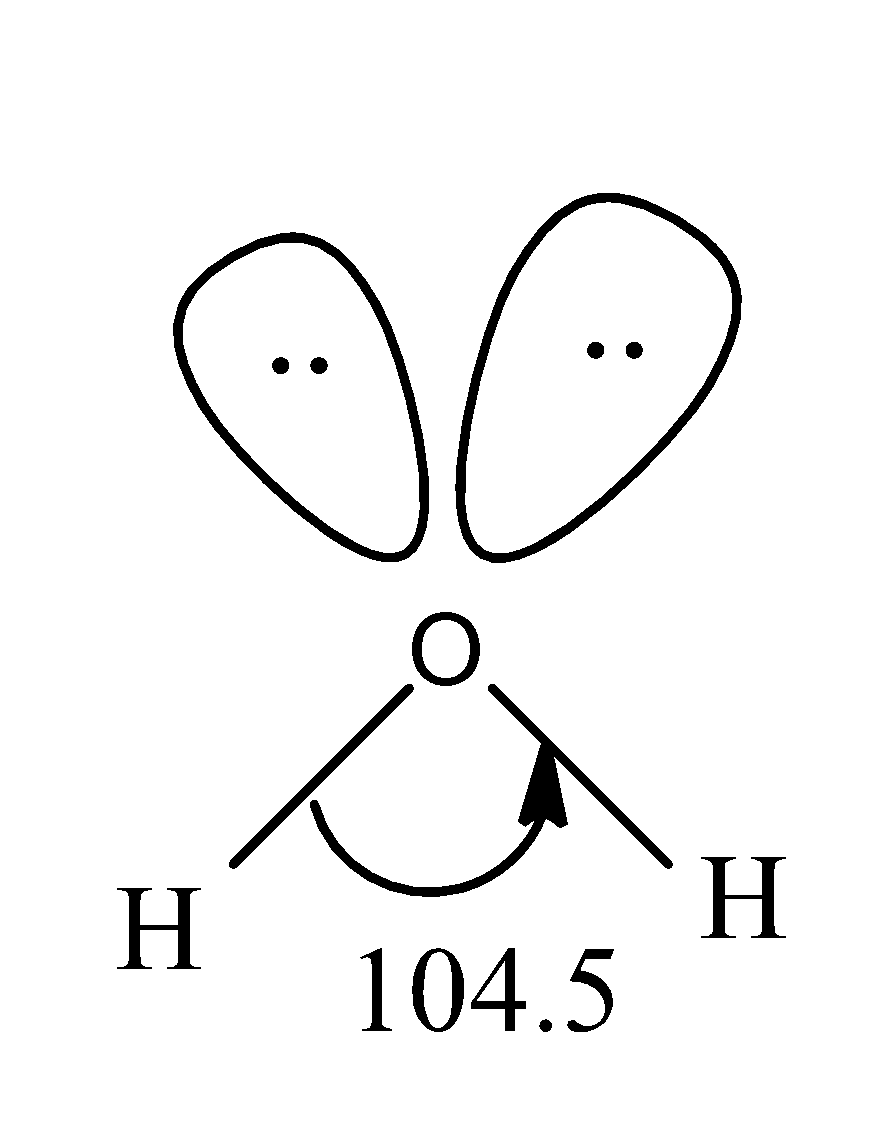
So, the $H-O-H$ angle in water molecules is about ${{104}^{0}}5'$.
So, the correct answer is “Option D”.
Note: The major disadvantage of the VSEPR theory is that it fails to determine the shapes and geometry for isoelectronic molecules and is also unable to explain why atomic orbital overlaps with each other.
Complete Solution :
- We can know the bond angle in water molecules through the VSEPR. VSEPR stands for the valence shell electron pair repulsion theory or simply we can say that VSEPR theory. This theory gives us information about the bond angles, bond length etc.
Now considering the statement:
In a water molecule, we know that there are two hydrogen atoms which are attached to the one oxygen atom with the oxygen atom being the central atom.
- The electronic configuration of the oxygen atom in the ground state is as 2, 6 i.e. it has 6 electrons in its outermost valence.
- The two hydrogen atoms contribute one electron each and therefore, the oxygen is left with a total of four electrons which are present in the form of two lone pairs.
- Therefore, for the coordination number 4 that is total of eight electrons or 4 electron pairs the hybridization of the water molecule is $s{{p}^{3}}$ and the geometry for this hybridization is tetrahedral and its bond angle should be ${{109}^{\circ }}{{5}^{'}}$ but due to lone pair repulsion, its bond angle reduces to ${{104}^{0}}5'$. So, thus, water has bent V-shaped structure and therefore is considered to have distorted tetrahedral geometry.
The structure of water molecule is as:

So, the $H-O-H$ angle in water molecules is about ${{104}^{0}}5'$.
So, the correct answer is “Option D”.
Note: The major disadvantage of the VSEPR theory is that it fails to determine the shapes and geometry for isoelectronic molecules and is also unable to explain why atomic orbital overlaps with each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




