
The hybridization of ${\left[ {Nic{l_4}} \right]^{2 - }}$ and ${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$ considering hybridization of the metal ion are respectively.
(A) $s{p^3},ds{p^2}$
(B) $ds{p^2},s{p^3}$
(C) Both $s{p^3}$
(D) Both $ds{p^2}$
Answer
584.1k+ views
Hint: According to valence bond theory, the metal atom or ion must possess a required number of vacant orbitals of equal energy . When hybridisation of the orbitals of metal atoms takes place to give a set of hybrid orbitals of equal energy , these orbitals are formed .
Complete step by step answer:
(A) ${\left[ {Nic{l_4}} \right]^{2 - }}$
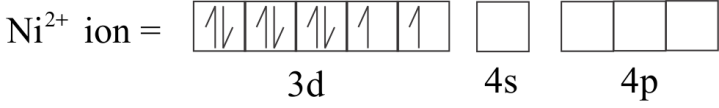
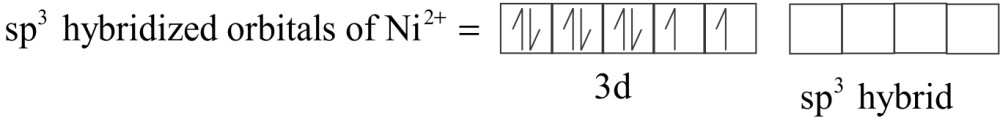
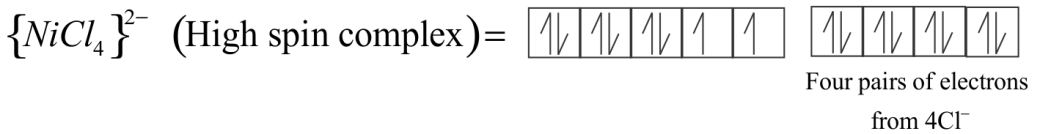
In tetrahedral complexes one ‘ $s$ ’and three ‘ $p$ ’ orbitals are hybridized to form four equivalent orbitals oriented tetrahedrally. Here, Nickel is in $ + 2$ oxidation state and the ion has two electronic configurations $3{d^8}$ . Each $C{l^ - }$ ion donates a pair of electrons. The compound is paramagnetic since it contains two unpaired electrons.
${\left[ {Nic{l_4}} \right]^{2 - }}$ has $s{p^3}$ Hybridization.
(B) ${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$
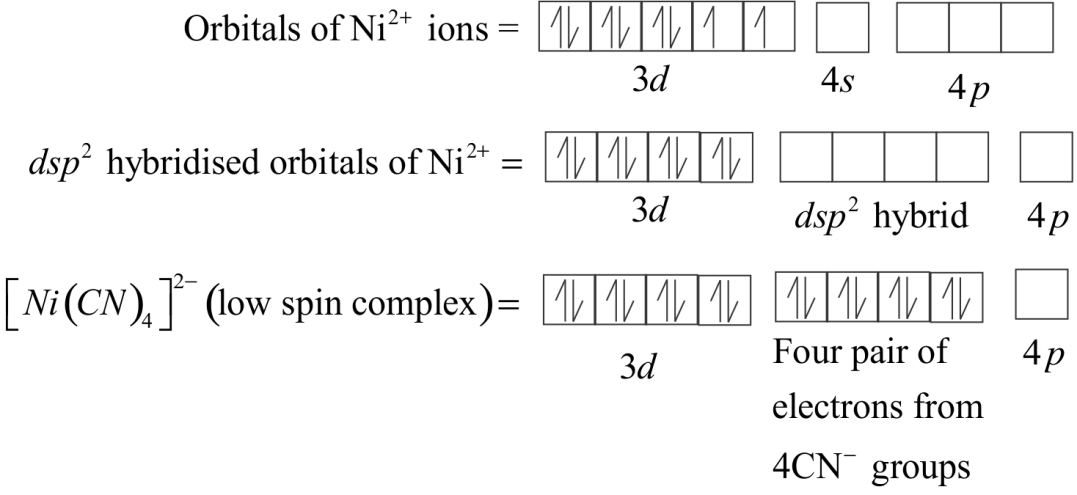
In the square planar complexes, Hybridization involved is $ds{p^2}$ . An example is ${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$ . Here nickel is in $ + 2$ oxidation state and has the electronic configuration $3{d^8}$ . The hybridization scheme is above.
Each of the hybridized orbitals receives a pair of electrons from a cyanide ion. The compound is diamagnetic as evident from the absence of unpaired electrons.
So the correct answer is A.
$s{p^3},ds{p^2}$.
Additional Information:
In VBT the basic assumption made is that the metal-ligand bond arises by the donation of pairs of electrons by ligands to the metal/ion. With the approach of ligands , metal-ligand bonds are then formed by the overlap of these orbitals with those of the ligands, i.e., by donation of electron pairs by the ligands of the empty hybridized orbitals. Consequently, these bonds are of equal strength and directional in nature.
Note:
Number of hybrid orbitals produced $ = $ number of hybrid involved in Hybridization
Valence bond theory is one of the earliest models of covalent bonding. This was proposed by
Pauling.
The transition metals have $s.p,d,f$ orbitals which undergo hybridization.
Complete step by step answer:
(A) ${\left[ {Nic{l_4}} \right]^{2 - }}$
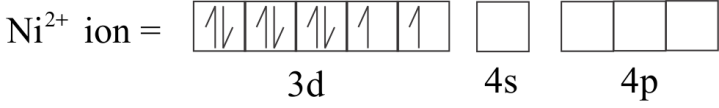
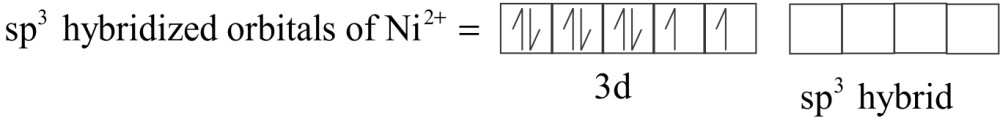
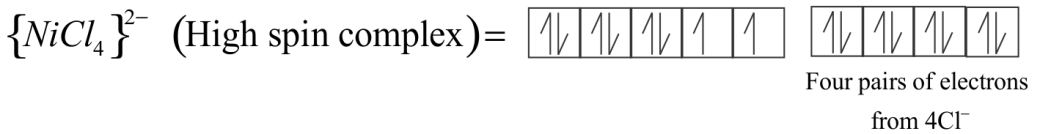
In tetrahedral complexes one ‘ $s$ ’and three ‘ $p$ ’ orbitals are hybridized to form four equivalent orbitals oriented tetrahedrally. Here, Nickel is in $ + 2$ oxidation state and the ion has two electronic configurations $3{d^8}$ . Each $C{l^ - }$ ion donates a pair of electrons. The compound is paramagnetic since it contains two unpaired electrons.
${\left[ {Nic{l_4}} \right]^{2 - }}$ has $s{p^3}$ Hybridization.
(B) ${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$

In the square planar complexes, Hybridization involved is $ds{p^2}$ . An example is ${\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$ . Here nickel is in $ + 2$ oxidation state and has the electronic configuration $3{d^8}$ . The hybridization scheme is above.
Each of the hybridized orbitals receives a pair of electrons from a cyanide ion. The compound is diamagnetic as evident from the absence of unpaired electrons.
So the correct answer is A.
$s{p^3},ds{p^2}$.
Additional Information:
In VBT the basic assumption made is that the metal-ligand bond arises by the donation of pairs of electrons by ligands to the metal/ion. With the approach of ligands , metal-ligand bonds are then formed by the overlap of these orbitals with those of the ligands, i.e., by donation of electron pairs by the ligands of the empty hybridized orbitals. Consequently, these bonds are of equal strength and directional in nature.
Note:
Number of hybrid orbitals produced $ = $ number of hybrid involved in Hybridization
Valence bond theory is one of the earliest models of covalent bonding. This was proposed by
Pauling.
The transition metals have $s.p,d,f$ orbitals which undergo hybridization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




