
The hybridization of the Chlorine atom in \[\text{Cl}{{\text{F}}_{\text{3}}}\] a molecule is:
(A) $sp$
(B) $s{{p}^{2}}$
(C) $s{{p}^{3}}$
(D) $s{{p}^{3}}d$
Answer
586.2k+ views
Hint: The hybridization is a process of mixing orbitals of varying energy to generate a set of degenerate orbitals. Here, in \[\text{Cl}{{\text{F}}_{\text{3}}}\] the chlorine is a central atom bonded to the three fluorine atoms by hybridization. The chlorine the s, p, and empty d orbital takes part in hybridization. This hybrid orbital results in the distorted trigonal bipyramidal or T-shaped geometry.
Complete step by step answer:
Chlorine trifluoride is a compound that has a molecular formula $\text{Cl}{{\text{F}}_{\text{3}}}$ and a bond angle of$\text{175}{}^\circ $. It has a t-shaped geometry. The following is the molecular structure of$\text{Cl}{{\text{F}}_{\text{3}}}$.
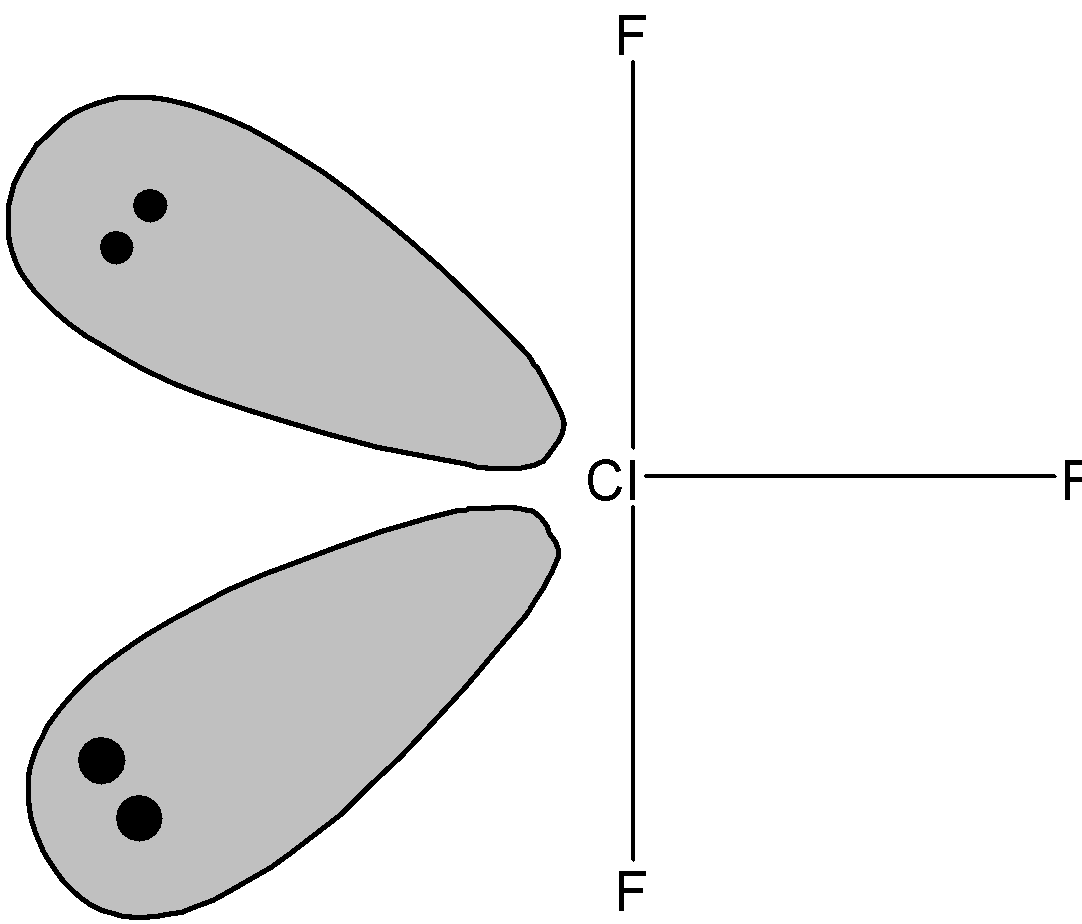
While talking about the hybridization of chlorine trifluoride, we usually consider its central atom that is chlorine. Chlorine contains 7 Valence Electrons. With this information, we can predict that chlorine trifluoride must consist of three bond pairs and two lone pairs.
The valence electronic configuration of chlorine is represented as \[\text{3}{{\text{s}}^{\text{2}}}\text{ 3}{{\text{p}}_{\text{x}}}^{\text{2}}\text{ 3}{{\text{p}}_{\text{y}}}^{\text{2}}\text{ 3}{{\text{p}}_{\text{z}}}^{\text{1}}\text{ 3d}\].
In chlorine trifluoride, we know that it needs three unpaired electrons in chlorine so that it can combine with fluorine atoms, but in this case, one of the electrons of chlorine in the 3p subshell remains unpaired. All the electrons in 3d orbitals participate in the process leading to the formation of 5 \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\] hybridization orbitals. In $\text{Cl}{{\text{F}}_{\text{3}}}$, 2 hybrid orbitals contain a pair of electrons while 3 hybrid orbitals contain unpaired electrons that overlap with the 2p orbitals of fluorine forming single bonds. The presence of two lone pairs in the chlorine trifluoride molecule results in a t-shaped geometry and an asymmetric charge distribution around chlorine which is the central atom of the molecule. The electron geometry of this compound is trigonal bipyramidal with the bond angle $\text{175}{}^\circ $.
Hence, the hybridization of the Chlorine atom in \[\text{Cl}{{\text{F}}_{\text{3}}}\] a molecule is \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\].
So, the correct answer is “Option D”.
Note: From local observation, we can say that \[\text{Cl}{{\text{F}}_{\text{3}}}\] is a trigonal planar molecule. But, one should always consider the lone pair on the central atom. Here, the two lone pairs act as part of a molecule. The lone pairs are considered to be nearer to the atom than the bond pairs, thus they have higher repulsive forces and destabilize the molecule. Thus, here the two lone pairs are placed in a plane which reduces the repulsion. However, the repulsion causes a decrease in the angle between the bond pair. This distorted trigonal bipyramidal geometry is called Bent T –shape.
Complete step by step answer:
Chlorine trifluoride is a compound that has a molecular formula $\text{Cl}{{\text{F}}_{\text{3}}}$ and a bond angle of$\text{175}{}^\circ $. It has a t-shaped geometry. The following is the molecular structure of$\text{Cl}{{\text{F}}_{\text{3}}}$.

While talking about the hybridization of chlorine trifluoride, we usually consider its central atom that is chlorine. Chlorine contains 7 Valence Electrons. With this information, we can predict that chlorine trifluoride must consist of three bond pairs and two lone pairs.
The valence electronic configuration of chlorine is represented as \[\text{3}{{\text{s}}^{\text{2}}}\text{ 3}{{\text{p}}_{\text{x}}}^{\text{2}}\text{ 3}{{\text{p}}_{\text{y}}}^{\text{2}}\text{ 3}{{\text{p}}_{\text{z}}}^{\text{1}}\text{ 3d}\].
In chlorine trifluoride, we know that it needs three unpaired electrons in chlorine so that it can combine with fluorine atoms, but in this case, one of the electrons of chlorine in the 3p subshell remains unpaired. All the electrons in 3d orbitals participate in the process leading to the formation of 5 \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\] hybridization orbitals. In $\text{Cl}{{\text{F}}_{\text{3}}}$, 2 hybrid orbitals contain a pair of electrons while 3 hybrid orbitals contain unpaired electrons that overlap with the 2p orbitals of fluorine forming single bonds. The presence of two lone pairs in the chlorine trifluoride molecule results in a t-shaped geometry and an asymmetric charge distribution around chlorine which is the central atom of the molecule. The electron geometry of this compound is trigonal bipyramidal with the bond angle $\text{175}{}^\circ $.
Hence, the hybridization of the Chlorine atom in \[\text{Cl}{{\text{F}}_{\text{3}}}\] a molecule is \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\].
So, the correct answer is “Option D”.
Note: From local observation, we can say that \[\text{Cl}{{\text{F}}_{\text{3}}}\] is a trigonal planar molecule. But, one should always consider the lone pair on the central atom. Here, the two lone pairs act as part of a molecule. The lone pairs are considered to be nearer to the atom than the bond pairs, thus they have higher repulsive forces and destabilize the molecule. Thus, here the two lone pairs are placed in a plane which reduces the repulsion. However, the repulsion causes a decrease in the angle between the bond pair. This distorted trigonal bipyramidal geometry is called Bent T –shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




