
The hybridization of Xe in $Xe{{O}_{2}}{{F}_{2}}$ and it’s shape are:
A.$s{{p}^{3}}{{d}^{2}}$, T shaped
B.$s{{p}^{3}}d$, see saw
C.$s{{p}^{3}}$, tetrahedral
D.$s{{p}^{3}}d$, V shaped
Answer
565.2k+ views
Hint: The shape of $A{{B}_{5}}$ molecule with zero lone pair is trigonal bipyramidal and with 4 bond pairs and one lone pair is T- shape and with 3 bond pair and 2 lone pairs is T- shape .We need to know about VSEPR theory for more details.
Complete step by step answer:
- VSEPR theory is defined as the electron pairs surrounding the atom present in the centre that must be arranged in space in such a distance so as to minimize the electrostatic repulsion experienced between them.
- The most important rule of the VSEPR theory states that the bond angles about a central atom are those that minimize the overall repulsion which is experienced between the Electron pairs in the valence shell of the atom.
- As the interior angle increases, the repulsion forces decrease sharply. As the value of electronegativity of an atom forming a molecule gets increased, the influence of a bonding electron pair decreases.
- Multiple bonds behave similar to a single electron pair for the sake of VSEPR bond theory.
- Hybridization of an atom can be found out by calculating the summation of lone pairs and sigma bonds.
If a sum of sigma bonds and lone pairs is 2, we have sp hybridization
If a sum of sigma bonds and lone pairs is 3 , we have $s{{p}^{2}}$ hybridization
If a sum of sigma bonds and lone pairs is 4 , we have $s{{p}^{3}}$ hybridization
If a sum of sigma bonds and lone pairs is 5, we have $s{{p}^{3}}d$ hybridization
If a sum of sigma bonds and lone pairs is 6, we have $s{{p}^{3}}{{d}^{2}}$ hybridization.
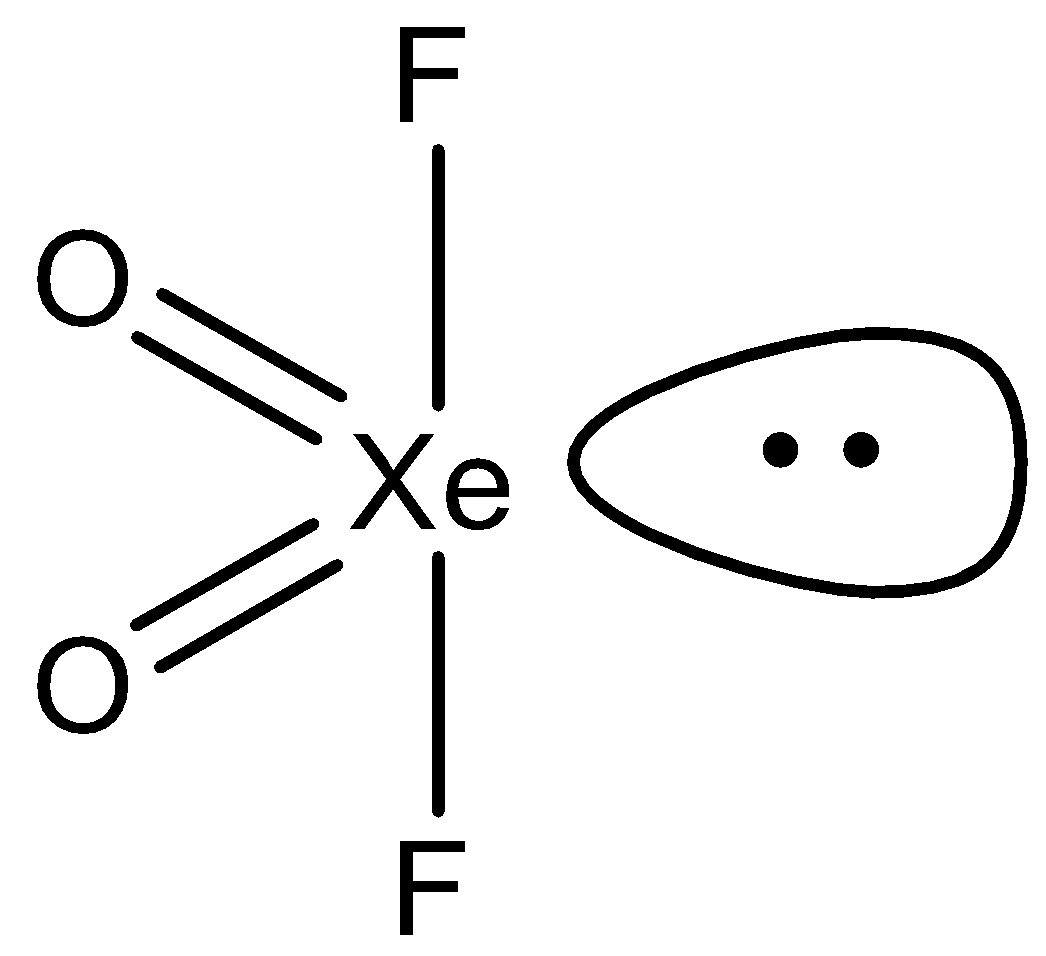
So the given molecule has 4 sigma bonds 1 lone pair that the sum is 5. So its hybridization is $s{{p}^{3}}d$ and therefore the shape is see saw.
So, the correct answer is “Option B”.
Note:
As a result of the distortions created different types of shapes can be drawn :
Complete step by step answer:
- VSEPR theory is defined as the electron pairs surrounding the atom present in the centre that must be arranged in space in such a distance so as to minimize the electrostatic repulsion experienced between them.
- The most important rule of the VSEPR theory states that the bond angles about a central atom are those that minimize the overall repulsion which is experienced between the Electron pairs in the valence shell of the atom.
- As the interior angle increases, the repulsion forces decrease sharply. As the value of electronegativity of an atom forming a molecule gets increased, the influence of a bonding electron pair decreases.
- Multiple bonds behave similar to a single electron pair for the sake of VSEPR bond theory.
- Hybridization of an atom can be found out by calculating the summation of lone pairs and sigma bonds.
If a sum of sigma bonds and lone pairs is 2, we have sp hybridization
If a sum of sigma bonds and lone pairs is 3 , we have $s{{p}^{2}}$ hybridization
If a sum of sigma bonds and lone pairs is 4 , we have $s{{p}^{3}}$ hybridization
If a sum of sigma bonds and lone pairs is 5, we have $s{{p}^{3}}d$ hybridization
If a sum of sigma bonds and lone pairs is 6, we have $s{{p}^{3}}{{d}^{2}}$ hybridization.

So the given molecule has 4 sigma bonds 1 lone pair that the sum is 5. So its hybridization is $s{{p}^{3}}d$ and therefore the shape is see saw.
So, the correct answer is “Option B”.
Note:
As a result of the distortions created different types of shapes can be drawn :
| Bond pair | Lone pair | Shape |
| 5 | 0 | Trigonal bipyramidal |
| 4 | 1 | See saw |
| 3 | 2 | T shape |
| 2 | 3 | Linear. |
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




