
The increasing order of rate of oxidation with \[HI{O_4}\] of the following is:
(I.) (II.)
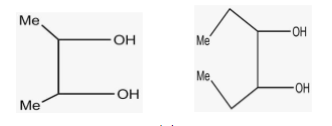
(III) (IV)
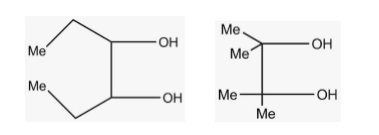
A.\[IV < {\text{ }}III{\text{ }} < {\text{ }}II{\text{ }} < {\text{ }}I\]
B.\[I{\text{ }} < {\text{ }}II{\text{ }} < {\text{ }}III{\text{ }} < {\text{ }}IV\]
C.\[IV{\text{ }} < {\text{ }}III{\text{ }} = {\text{ }}II{\text{ }} < {\text{ }}I\]
D.\[I{\text{ }} < {\text{ }}II{\text{ }} < {\text{ }}III{\text{ }} < {\text{ }}IV\]


Answer
588k+ views
Hint: \[HI{O_4}\] reacts with vicinal diols (compound having 2 alcohols attached). It is a nucleophilic reaction and water is removed as a byproduct. More hindrance in the compound will decrease the rate of reaction. So, for the reaction to be at a faster rate less bulky should be attached to the carbon.
Complete step by step answer:
More the steric hindrance, lesser is the oxidation. The correct increasing order for oxidation according to the figure is \[\;IV < {\text{ }}III{\text{ }} < {\text{ }}II{\text{ }} < {\text{ }}I\] as in fig.I .There is no bulky group present.
1, 2 or vicinal diols are cleaved by periodic acid, \[HI{O_4}\] into two carbonyl compounds. The ester undergoes rearrangement of the electrons, cleavage of \[C-C\] bond and forming two \[C = O.\;\]
-The heavier the diol less chance to undergo oxidation by \[HI{O_4}\].
-This option is absolutely correct according to the question because there is no bulkier group attached to the compound of fig I. so, there is no steric hindrance and rate of oxidation is fast in fig I.
-This option is not correct as it shows the reverse of the correct answer. Heavier diol is shown in fig IV so the oxidation is less. But in this option, oxidation is shown more in fog IV.
-This option shows that the rate of oxidation is equal for III and I which is not correct at all as it does go in the favour of what we studied. So, this option is incorrect.
-This option also does not go correct with the question because it shows that rate of oxidation is equal for II and III in fact the order is also not right.
Note:
Vicinal diols are cleaved by periodic acid to yield aldehydes or ketones depending on the number of substituents on the carbon atoms. Diols provides an alternative method to ozonolysis of alkenes. Steric hindrance is the retardation of a chemical reaction caused by the arrangement of atoms in a molecule.
Complete step by step answer:
More the steric hindrance, lesser is the oxidation. The correct increasing order for oxidation according to the figure is \[\;IV < {\text{ }}III{\text{ }} < {\text{ }}II{\text{ }} < {\text{ }}I\] as in fig.I .There is no bulky group present.
1, 2 or vicinal diols are cleaved by periodic acid, \[HI{O_4}\] into two carbonyl compounds. The ester undergoes rearrangement of the electrons, cleavage of \[C-C\] bond and forming two \[C = O.\;\]
-The heavier the diol less chance to undergo oxidation by \[HI{O_4}\].
-This option is absolutely correct according to the question because there is no bulkier group attached to the compound of fig I. so, there is no steric hindrance and rate of oxidation is fast in fig I.
-This option is not correct as it shows the reverse of the correct answer. Heavier diol is shown in fig IV so the oxidation is less. But in this option, oxidation is shown more in fog IV.
-This option shows that the rate of oxidation is equal for III and I which is not correct at all as it does go in the favour of what we studied. So, this option is incorrect.
-This option also does not go correct with the question because it shows that rate of oxidation is equal for II and III in fact the order is also not right.
Note:
Vicinal diols are cleaved by periodic acid to yield aldehydes or ketones depending on the number of substituents on the carbon atoms. Diols provides an alternative method to ozonolysis of alkenes. Steric hindrance is the retardation of a chemical reaction caused by the arrangement of atoms in a molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




