
The intramolecular hydrogen bond is present in:
A. Phenol
B. o-nitrophenol
C. p-nitrophenol
D. p-Cresol
Answer
601.2k+ views
Hint: To answer this question we should know about intramolecular hydrogen bonds.The hydrogen bonding will occur only when a hydrogen atom is bonded to a strongly electronegative atom which then exists in the neighbour of another electronegative atom with a lone pair of electrons.
Step by step answer:
If in a molecule, there is hydrogen bonding then we should know that there must be the presence of both a hydrogen donor and an acceptor. Now, we will know about the donor in a hydrogen bond. It is the atom to which the hydrogen atom participating in the hydrogen bond is covalently bonded, and is usually a strongly electronegative atom such as nitrogen, oxygen and fluorine. And the part which is a hydrogen acceptor is the neighbouring electronegative ion or molecule, and must possess a lone electron pair in order to form a hydrogen bond.
Now, we will know about intramolecular hydrogen bonding:
Intramolecular hydrogen bonds: We should know that intramolecular hydrogen bonding occurs in one single molecule. It should be noted that, if it occurs on one single molecule then both a hydrogen donor and acceptor must be present within one molecule.
So, now we know the basic concept about intramolecular hydrogen bonding. Now, we are ready to select the option which will show intramolecular hydrogen bonding.
First option is Phenol.
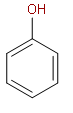
The above structure is of phenol. Phenol does not show intramolecular hydrogen bonding. We have the donor molecule attached to the benzene ring, but there is no acceptor molecule to do hydrogen bonding with the donor molecule. The presence of both donor and acceptor molecules on the same structure is the essential condition for intramolecular hydrogen bonding.
Now, we will take the second option.
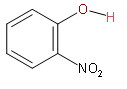
The above structure is o-nitrophenol. As both the donor as well as acceptor molecule are present on the same molecule then there is possibility of intramolecular hydrogen bonding. Next condition for intramolecular hydrogen bonding is that there must be the presence of a lone pair on the acceptor molecule atom. So, oxygen in the nitro group has lone pairs.
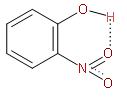
The above structure is of o-nitrophenol showing hydrogen bonding.
Now, we will check the third option whether it has intramolecular hydrogen bonding or not.

The above structure is of p-nitrophenol. As we can see, both donor and acceptor molecules are present on the same molecule but they are very far from each other. So, hydrogen bonding is not possible.
Now, we will look at the fourth option.

The above structure is of p-cresol. We should note that there is not any acceptor part present. There is a methyl group present and it doesn’t have lone pairs to accept electrons.
So, from the discussion we can say that option B is correct. o-Nitrophenol will show hydrogen bonding.
Note:
So, let us now discuss the second type of hydrogen bonding that is intermolecular hydrogen bonding. We should note that intermolecular hydrogen bonds occur between separate molecules in a substance. We should note that intermolecular hydrogen bonding can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present and in positions in which they can interact. For example, intermolecular hydrogen bonds can occur between \[N{{H}_{3}}\] molecules alone, between \[{{H}_{2}}O\] molecules alone, or between \[N{{H}_{3}}\]and \[{{H}_{2}}O\] molecule.
Step by step answer:
If in a molecule, there is hydrogen bonding then we should know that there must be the presence of both a hydrogen donor and an acceptor. Now, we will know about the donor in a hydrogen bond. It is the atom to which the hydrogen atom participating in the hydrogen bond is covalently bonded, and is usually a strongly electronegative atom such as nitrogen, oxygen and fluorine. And the part which is a hydrogen acceptor is the neighbouring electronegative ion or molecule, and must possess a lone electron pair in order to form a hydrogen bond.
Now, we will know about intramolecular hydrogen bonding:
Intramolecular hydrogen bonds: We should know that intramolecular hydrogen bonding occurs in one single molecule. It should be noted that, if it occurs on one single molecule then both a hydrogen donor and acceptor must be present within one molecule.
So, now we know the basic concept about intramolecular hydrogen bonding. Now, we are ready to select the option which will show intramolecular hydrogen bonding.
First option is Phenol.

The above structure is of phenol. Phenol does not show intramolecular hydrogen bonding. We have the donor molecule attached to the benzene ring, but there is no acceptor molecule to do hydrogen bonding with the donor molecule. The presence of both donor and acceptor molecules on the same structure is the essential condition for intramolecular hydrogen bonding.
Now, we will take the second option.

The above structure is o-nitrophenol. As both the donor as well as acceptor molecule are present on the same molecule then there is possibility of intramolecular hydrogen bonding. Next condition for intramolecular hydrogen bonding is that there must be the presence of a lone pair on the acceptor molecule atom. So, oxygen in the nitro group has lone pairs.

The above structure is of o-nitrophenol showing hydrogen bonding.
Now, we will check the third option whether it has intramolecular hydrogen bonding or not.

The above structure is of p-nitrophenol. As we can see, both donor and acceptor molecules are present on the same molecule but they are very far from each other. So, hydrogen bonding is not possible.
Now, we will look at the fourth option.

The above structure is of p-cresol. We should note that there is not any acceptor part present. There is a methyl group present and it doesn’t have lone pairs to accept electrons.
So, from the discussion we can say that option B is correct. o-Nitrophenol will show hydrogen bonding.
Note:
So, let us now discuss the second type of hydrogen bonding that is intermolecular hydrogen bonding. We should note that intermolecular hydrogen bonds occur between separate molecules in a substance. We should note that intermolecular hydrogen bonding can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present and in positions in which they can interact. For example, intermolecular hydrogen bonds can occur between \[N{{H}_{3}}\] molecules alone, between \[{{H}_{2}}O\] molecules alone, or between \[N{{H}_{3}}\]and \[{{H}_{2}}O\] molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




