
The IUPAC name of $C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}$.
A. Ethyl acetate
B. Ethyl ethanoate
C. Methyl propanoate
D. None of the above
Answer
565.2k+ views
Hint: IUPAC gives us some rules for the naming of organic compounds which can also be referred to as nomenclature of organic compounds. IUPAC stands for International Union of Pure and Applied Chemistry.
Complete answer:
Ethyl acetate is the common name of the compound and it can be represented by the molecular formula $C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}$which can also be shown as:
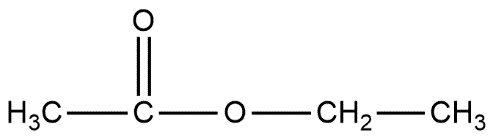
IUPAC nomenclature gives us information on writing the name of chemical compounds. There are some general rules for it. To write the name of this compound we have to follow some rules.
a. At first select the longest chain of carbon atoms, in the given compound the longest chain is of 4 carbon atoms from right to left.
b. Now we see there is a carbonyl group present in the compound and the compound represents the formula in the form of $R-COO-R'$ which represents ester.
c. In the given compound $R=C{{H}_{3}}$ and $R'=C{{H}_{2}}C{{H}_{3}}$.
d. In this $C{{H}_{2}}C{{H}_{3}}$ is named as ethyl and another group is taken along with $COO$i.e. $C{{H}_{3}}COO$ known by the name ethanoate. Hence the IUPAC name of the compound will be ethyl ethanoate.
Hence option B is the correct answer.
Note:
The main aim of the nomenclature IUPAC is to create an international standard for designating compounds to promote communication. The purpose of the system is to give a unique and unambiguous name to each structure so that no two structures' names get mixed or they can be identified easily.
Complete answer:
Ethyl acetate is the common name of the compound and it can be represented by the molecular formula $C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}$which can also be shown as:

IUPAC nomenclature gives us information on writing the name of chemical compounds. There are some general rules for it. To write the name of this compound we have to follow some rules.
a. At first select the longest chain of carbon atoms, in the given compound the longest chain is of 4 carbon atoms from right to left.
b. Now we see there is a carbonyl group present in the compound and the compound represents the formula in the form of $R-COO-R'$ which represents ester.
c. In the given compound $R=C{{H}_{3}}$ and $R'=C{{H}_{2}}C{{H}_{3}}$.
d. In this $C{{H}_{2}}C{{H}_{3}}$ is named as ethyl and another group is taken along with $COO$i.e. $C{{H}_{3}}COO$ known by the name ethanoate. Hence the IUPAC name of the compound will be ethyl ethanoate.
Hence option B is the correct answer.
Note:
The main aim of the nomenclature IUPAC is to create an international standard for designating compounds to promote communication. The purpose of the system is to give a unique and unambiguous name to each structure so that no two structures' names get mixed or they can be identified easily.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




