
The IUPAC name of compound?
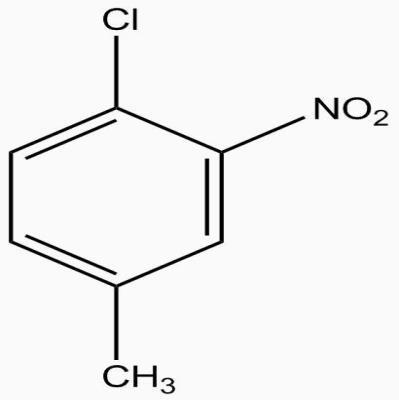
A.1-Chloro-2 nitro-4-methylbenzene
B. 1-chloro-4 methyl -2nitrobenzene
C. 2-Chloro-1 nitro-5- methylbenzene
D. m-nitro-p-chlorotoluene

Answer
586.2k+ views
Hint: Nomenclature means naming of organic compounds.
It is written as
Secondary prefix + primary prefix + word root + primary suffix + secondary suffix.
Complete step by step answer:
First we select the longest chain of hydrocarbon that is 6 carbon chain benzene.
Now functional groups attached to it are given numbers according to their alphabetical order.
The alphabet that comes first will be written in naming first like first chlorine then methyl and then nitro
If we will first number methyl group starting from chlorine the $\mathop {NO}\nolimits_2 $ occupy $\mathop 6\nolimits^{th} $ position but if we first give number to $\mathop {NO}\nolimits_2 $ group methyl will remain on same position that is 4 and nitro will occupy second position which is in accordance with rule of nomenclature.
Now for chlorine group we use Chloro
For $\mathop {CH}\nolimits_3 $ we use methyl
For $\mathop {NO}\nolimits_2 $ we use nitro as a prefix.
Now according to the following rules the compound will get name
1-chloro-4-methyl-2nitrobenzene
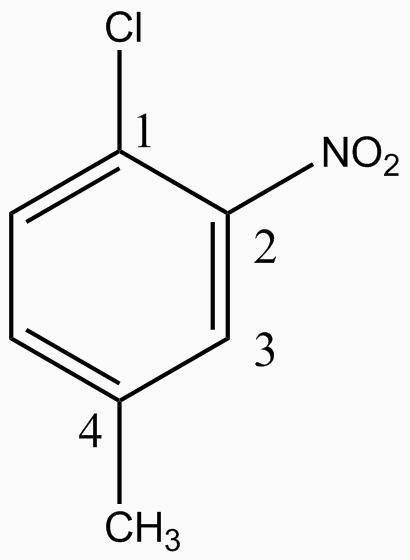
Now let us match the above stated rules with given option:-
Option A :- in this option the nitro should be named after methyl as alphabet n comes later . So this is against the rules of nomenclature. Hence this option is not correct.
Option B :- this option follows every rule of nomenclature as longest chain rule and alphabetical rule. So this option is correct.
Option C :- the number to the groups in the benzene ring is not according to nomenclature rule as they are not following the smallest number rule. Thus this option is not correct. For example chloro can occupy 1st position.
Option D :- the chlorine group is not at para position to the nitro group, that is it is not opposite to it. Hence this option is not correct.
Our required answer, is b that 1-chloro-4-methyl-2nitrobenzene.
Note:
Functional group is defined as atom or group of atoms bonded together in a unique manner present in the molecule which largely determines its chemical properties as in above compound nitro , methyl and chlorine were functional groups.
It is written as
Secondary prefix + primary prefix + word root + primary suffix + secondary suffix.
Complete step by step answer:
First we select the longest chain of hydrocarbon that is 6 carbon chain benzene.
Now functional groups attached to it are given numbers according to their alphabetical order.
The alphabet that comes first will be written in naming first like first chlorine then methyl and then nitro
If we will first number methyl group starting from chlorine the $\mathop {NO}\nolimits_2 $ occupy $\mathop 6\nolimits^{th} $ position but if we first give number to $\mathop {NO}\nolimits_2 $ group methyl will remain on same position that is 4 and nitro will occupy second position which is in accordance with rule of nomenclature.
Now for chlorine group we use Chloro
For $\mathop {CH}\nolimits_3 $ we use methyl
For $\mathop {NO}\nolimits_2 $ we use nitro as a prefix.
Now according to the following rules the compound will get name
1-chloro-4-methyl-2nitrobenzene

Now let us match the above stated rules with given option:-
Option A :- in this option the nitro should be named after methyl as alphabet n comes later . So this is against the rules of nomenclature. Hence this option is not correct.
Option B :- this option follows every rule of nomenclature as longest chain rule and alphabetical rule. So this option is correct.
Option C :- the number to the groups in the benzene ring is not according to nomenclature rule as they are not following the smallest number rule. Thus this option is not correct. For example chloro can occupy 1st position.
Option D :- the chlorine group is not at para position to the nitro group, that is it is not opposite to it. Hence this option is not correct.
Our required answer, is b that 1-chloro-4-methyl-2nitrobenzene.
Note:
Functional group is defined as atom or group of atoms bonded together in a unique manner present in the molecule which largely determines its chemical properties as in above compound nitro , methyl and chlorine were functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




