
The IUPAC name of is:
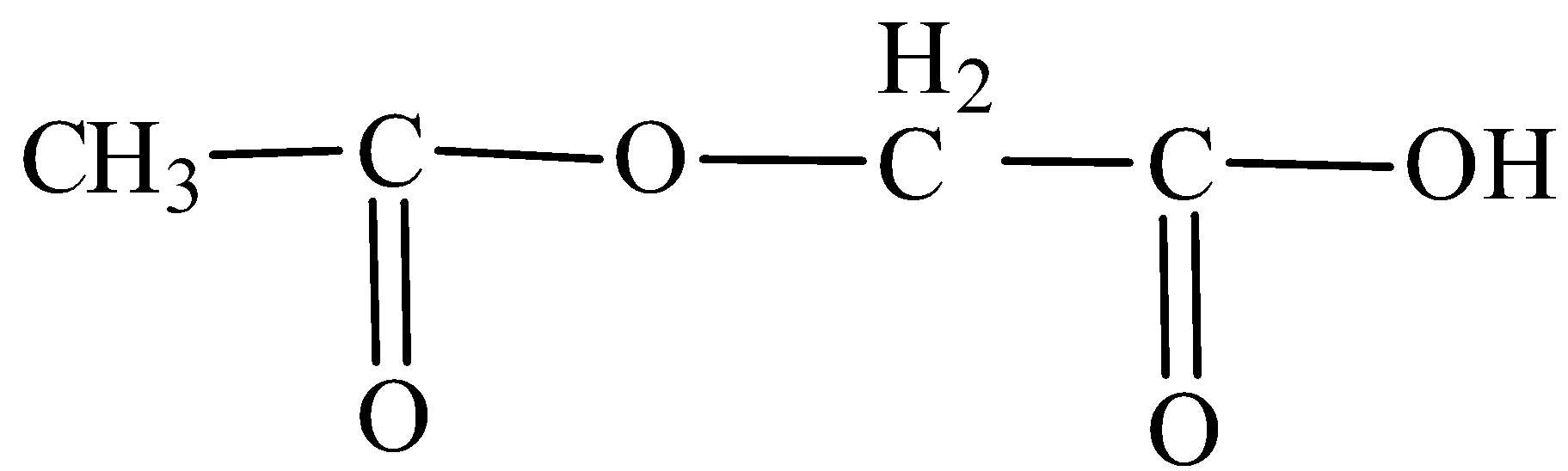
A.1-Acetoxy acetic acid
B.2-Acetoxy ethanoic acid
C.2-Ethanoyloxy acetic acid
D.2-Ethanoyloxyethanoic acid
Answer
510.3k+ views
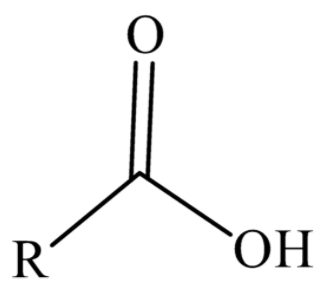
Hint: We have to know that carboxylic compound is an organic compound that has a carboxyl functional group. We have to know that the general representation of carboxylic acid is $R - COOH$, here R represents alkyl group and $COOH$ represents carboxyl group. We have to draw the general structure of carboxylic acid is,
Complete answer:
We know that the general structure of carboxylic acid has $R - COOH$. In the carboxyl group, the atom of carbon shares a double bond with an atom of oxygen, and carbon shares a single bond with the hydroxyl group. The carboxyl functional group would have a carbonyl functional group $\left( {CO} \right)$ and a hydroxyl functional group $\left( {OH} \right)$. Because of the electronegativity found in atoms of oxygen, the functional group could undergo ionization and protons are discharged.
We have a set of rules for naming IUPAC of carboxylic acid. We can write the rules as:
We have to determine the parent compound. The parent compound is nothing but the longest continuous chain of carbon which bears the carboxyl group.
We have to number the chain that has carboxyl carbon as C-1.
We have to replace the “-e” ending of alkane with the suffix “-oic acid”.
Then, we have to name and number the substituents found in the compound.
We are given the structure of the compound as,
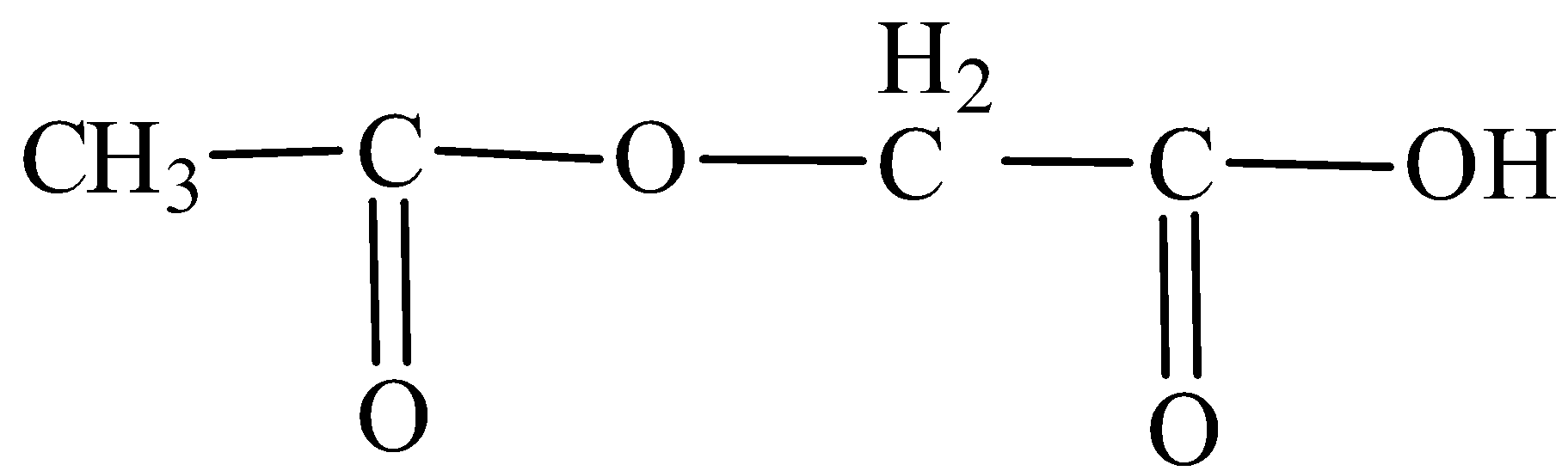
We have to know that the parent compound is ethane. So, the name of the parent compound becomes ethanoic acid. In the carbon second position, the substituent is acetoxy group. So, the IUPAC name of the given structure is 2-acetoxyethanoic acid.
Option (b) is correct.
Note:
We have to know that in case two carboxyl groups are present in the compound, then the suffix would be “-dioic acid”. In case if we have hydroxyl group as one of the substituents, then we have to number the hydroxyl group and we can use the word “hydroxy” as prefix.

Complete answer:
We know that the general structure of carboxylic acid has $R - COOH$. In the carboxyl group, the atom of carbon shares a double bond with an atom of oxygen, and carbon shares a single bond with the hydroxyl group. The carboxyl functional group would have a carbonyl functional group $\left( {CO} \right)$ and a hydroxyl functional group $\left( {OH} \right)$. Because of the electronegativity found in atoms of oxygen, the functional group could undergo ionization and protons are discharged.
We have a set of rules for naming IUPAC of carboxylic acid. We can write the rules as:
We have to determine the parent compound. The parent compound is nothing but the longest continuous chain of carbon which bears the carboxyl group.
We have to number the chain that has carboxyl carbon as C-1.
We have to replace the “-e” ending of alkane with the suffix “-oic acid”.
Then, we have to name and number the substituents found in the compound.
We are given the structure of the compound as,
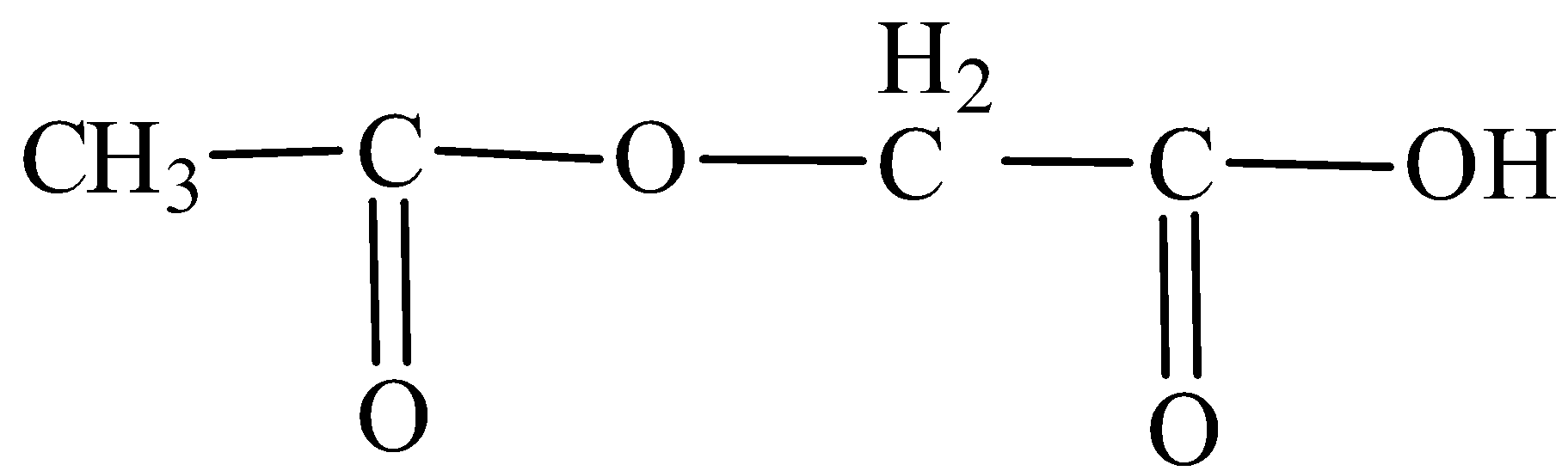
We have to know that the parent compound is ethane. So, the name of the parent compound becomes ethanoic acid. In the carbon second position, the substituent is acetoxy group. So, the IUPAC name of the given structure is 2-acetoxyethanoic acid.
Option (b) is correct.
Note:
We have to know that in case two carboxyl groups are present in the compound, then the suffix would be “-dioic acid”. In case if we have hydroxyl group as one of the substituents, then we have to number the hydroxyl group and we can use the word “hydroxy” as prefix.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




