
The IUPAC name of sec.butyl alcohol is:
A. 1-butanol
B. 2-butanol
C. 2-methyl-1-butanol
D. 2-methyl-2-butanol
Answer
570k+ views
Hint: The prefixes that is sec (from “secondary”), tert (from “tertiary”) etc. denotes the position of the functional groups that are present in the given compound or the additional side chains connected at that position.
Where sec = 2 , Tert = 3
Complete Solution :
Given sec.butyl alcohol is the compound
In which the functional group is alcohol $\left( -OH \right)$ which is at the position of second carbon.
The structure of the compound is as follows:
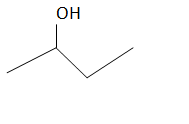
There are four carbons in this structure and alcohol functional group is at the second carbon which is having the IUPAC name as
2-butanol or butan-2-ol
Hence the option (B) is correct
- In option (A) the functional group is the position of first carbon which does not matches with the given compound hence it is not the correct option
- In option (C) the functional group is the position of first carbon and also an extra methyl group is added at the second which does not matches with the given compound hence it is not the correct option
- In option (D) the functional group is the position of second carbon and also an extra methyl group is added at the second which does not match with the given compound hence it is not the correct option.
So, the correct answer is “Option B”.
Note: It will be easy to write the IUPAC name if we draw the structure of the compound given. The full form of IUPAC is the International Union of Pure and Applied Chemistry. The IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry. The names of the organic chemical compounds sometimes will write in simpler form as given in the question, that is sec. butyl alcohol.
Where sec = 2 , Tert = 3
Complete Solution :
Given sec.butyl alcohol is the compound
In which the functional group is alcohol $\left( -OH \right)$ which is at the position of second carbon.
The structure of the compound is as follows:

There are four carbons in this structure and alcohol functional group is at the second carbon which is having the IUPAC name as
2-butanol or butan-2-ol
Hence the option (B) is correct
- In option (A) the functional group is the position of first carbon which does not matches with the given compound hence it is not the correct option
- In option (C) the functional group is the position of first carbon and also an extra methyl group is added at the second which does not matches with the given compound hence it is not the correct option
- In option (D) the functional group is the position of second carbon and also an extra methyl group is added at the second which does not match with the given compound hence it is not the correct option.
So, the correct answer is “Option B”.
Note: It will be easy to write the IUPAC name if we draw the structure of the compound given. The full form of IUPAC is the International Union of Pure and Applied Chemistry. The IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry. The names of the organic chemical compounds sometimes will write in simpler form as given in the question, that is sec. butyl alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




