
The IUPAC name of tertiary butyl chloride is-
A. 2-chloro-2-methylpropane
B. 3-chlorobutane
C. 4-chlorobutane
D. 1, 2-chloro-3-methylpropane.
Answer
601.5k+ views
Hint- This question demands us to find the IUPAC name of the compound name tertiary butyl chloride. To find the naming we need to Recognize the functional group in the compound, Look for the longest continuous carbon chain (it won't always be straight) and count the number of carbon atoms in this chain. Number the carbons in the longest carbon chain (important: if the molecule does no longer have an alkane (i.e. a functional group) the numbers should begin to be numbered in order to have the lowest number of functional groups in the carbon). At the end closest to the functional group, begin with the carbon. We need to look for the branched group too. For the alkyl halides the halogen atom is treated in much the same way as branched groups.
Complete step-by-step answer:
IUPAC name of tertiary butyl chloride
Step one: Identify the functional group
Step two: Find the longest carbon chain
Step three: Number the carbon atoms in the longest chain
Step four: Look for any branched group, name them and give their position on the carbon chain
Step five: Combine the elements of the name into a single word
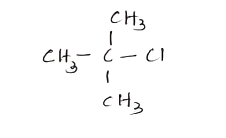
Hence, IUPAC name of tertiary butyl chloride is 2- chloro-2-methyl-propane.
Note- We should know the basic IUPAC naming rules in order to find the IUPAC names of the required organic compounds. We could easily solve this question if we knew the structure of the tertiary butyl- compound. Tertiary stands for three. This gives us the hint that there would be three methyl groups surrounding the carbon.
Complete step-by-step answer:
IUPAC name of tertiary butyl chloride
Step one: Identify the functional group
Step two: Find the longest carbon chain
Step three: Number the carbon atoms in the longest chain
Step four: Look for any branched group, name them and give their position on the carbon chain
Step five: Combine the elements of the name into a single word
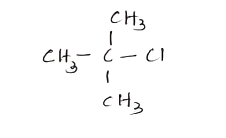
Hence, IUPAC name of tertiary butyl chloride is 2- chloro-2-methyl-propane.
Note- We should know the basic IUPAC naming rules in order to find the IUPAC names of the required organic compounds. We could easily solve this question if we knew the structure of the tertiary butyl- compound. Tertiary stands for three. This gives us the hint that there would be three methyl groups surrounding the carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




