
The IUPAC name of the compound is:
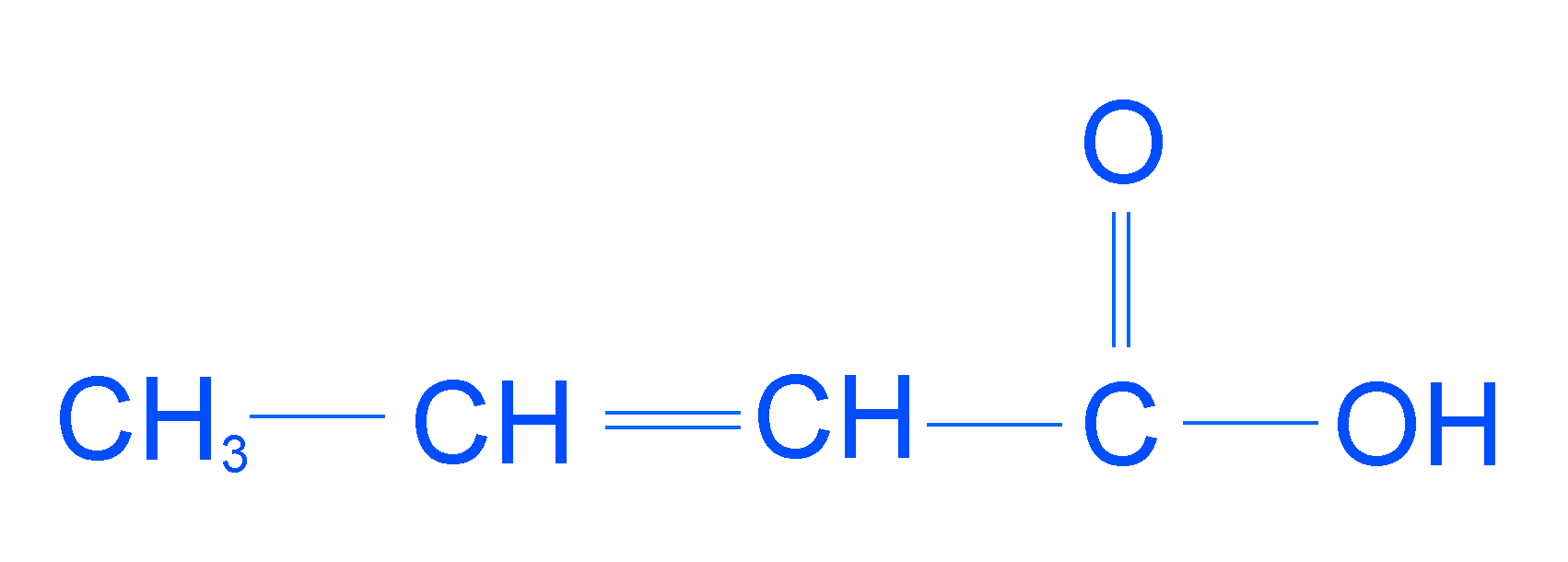
a.) But-1-en-4-oic acid
b.) 1-hydroxybut-2-en-1-one
c.) But-2-en-1-oic acid
d.) But-2-en-4-oic acid
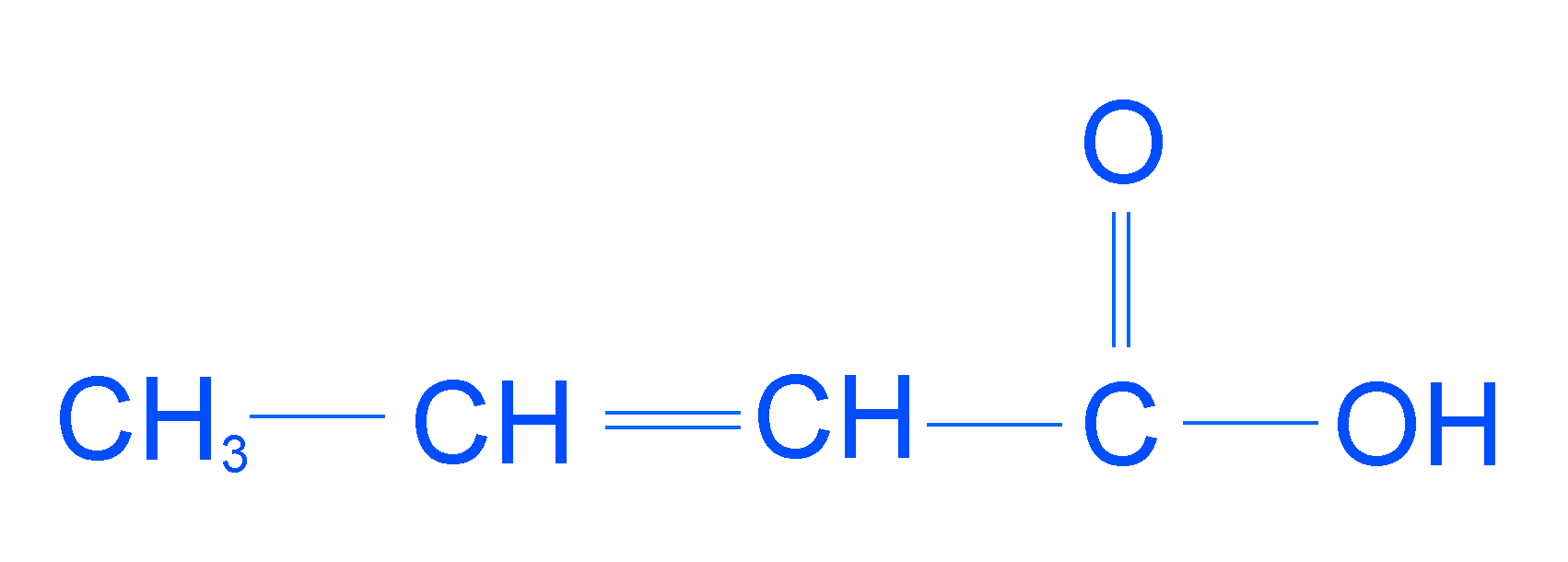
Answer
584.4k+ views
Hint: Count the longest chain of carbon, then give the lowest possible number to the carbon with the carboxylic group and then the second-lowest number to the carbon with a double bond. Following these rules, we name the compound But-2-en-1-oic acid.
Complete step by step answer:
To solve this problem we have to first know some basic rules of IUPAC (International Union of Pure and Applied Chemistry)
Some rules of IUPAC nomenclature of compounds containing carboxylic or carboxyl acid and double bonds are:
Count the longest continuous chain of carbon atoms possible (that can contain the carbon atom of the carboxylic group).
In the numbering of the parent chain, the carboxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds.
Suffix oic-acid is added when the carboxylic group is present in the main chain (also called the parent chain)
The -en suffix immediately follows the parent chain when both double bonds and carboxyl groups are present, and the-oic acid suffix follows the -en suffix (Note that the e is left off, -en rather than –ene).
The position of the double bond(s) is indicated before the parent name, and the- en suffix is immediately followed by the -oic acid suffix. Note that the position of the carboxyl group need not be specified, since it will naturally be carbon 1.
Now let’s follow the rules and find out the IUPAC name of the given compound
Following step 1 the longest continuous chain of carbon atom will be of 4 carbon atoms.
Following steps 2, 3, and 4 we see that the carboxylic group is in the first position and double bond is in the second position.
So, the IUPAC name of the given compound is But-2-en-1-oic acid.
So, the correct answer is “Option C”.
Note: As we saw in the solution above that the position of the carboxylic group is prioritized over the position of the alkene group (meaning the carboxylic group is given a smaller number than the alkene group). This was done based on a priority order that is provided by IUPAC, this priority order is always followed when naming any compound.
Complete step by step answer:
To solve this problem we have to first know some basic rules of IUPAC (International Union of Pure and Applied Chemistry)
Some rules of IUPAC nomenclature of compounds containing carboxylic or carboxyl acid and double bonds are:
Count the longest continuous chain of carbon atoms possible (that can contain the carbon atom of the carboxylic group).
In the numbering of the parent chain, the carboxyl group takes precedence over alkyl groups and halogen substituents, as well as double bonds.
Suffix oic-acid is added when the carboxylic group is present in the main chain (also called the parent chain)
The -en suffix immediately follows the parent chain when both double bonds and carboxyl groups are present, and the-oic acid suffix follows the -en suffix (Note that the e is left off, -en rather than –ene).
The position of the double bond(s) is indicated before the parent name, and the- en suffix is immediately followed by the -oic acid suffix. Note that the position of the carboxyl group need not be specified, since it will naturally be carbon 1.
Now let’s follow the rules and find out the IUPAC name of the given compound
Following step 1 the longest continuous chain of carbon atom will be of 4 carbon atoms.
Following steps 2, 3, and 4 we see that the carboxylic group is in the first position and double bond is in the second position.
So, the IUPAC name of the given compound is But-2-en-1-oic acid.
So, the correct answer is “Option C”.
Note: As we saw in the solution above that the position of the carboxylic group is prioritized over the position of the alkene group (meaning the carboxylic group is given a smaller number than the alkene group). This was done based on a priority order that is provided by IUPAC, this priority order is always followed when naming any compound.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




