
The IUPAC name of the following compound is:
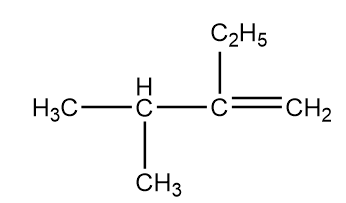
A. 3-Ethyl-2-methylbut-3-ene
B. 2-Ethyl-3-methylbut-1-ene
C. 2-Methyl-3-ethylbut-3-ene
D. 3-Methyl-2-ethylbut-1-ene

Answer
497.7k+ views
Hint: In IUPAC nomenclature, the rational system must have at least two things i.e., first it should indicate that how the carbon atoms of a given compound are bonded together in a characteristic lattice of rings and chains and secondly, it should identify and locate any functional group (if present), in the compound.
Complete Step By Step Answer:
As per the question, the given compound is an alkene and IUPAC rules for naming alkene and cycloalkene are as follows:
1. The name should contain an ene suffix i.e., the IUPAC name ending with “ene” represents alkene or cycloalkene.
2. The longest chain chosen for the base or root must contain both carbon atoms of the double bond.
3. The chain must be numbered in such a way that the double bond gets minimum allocation i.e., it must be numbered from the end nearest the doubly bonded carbon atom.
4. If in case, more than one double bond is present in the compound then the compound is named as diene, triene and so on.
5. The names of functional groups are always written in alphabetical order.
According to these rules, the numbering for the given compound will be as follows:
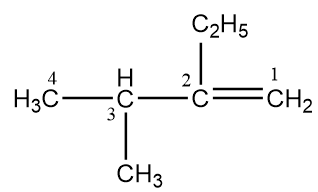
In the compound, an ethyl group is present at the second position and at third position, a methyl group is present. Hence, the IUPAC name of the given compound is 2-Ethyl-3-methylbut-1-ene.
Thus, option (B) is the correct answer.
Note:
Remember that we can recognize organic compounds from their common names as well. The IUPAC names strictly follow an internationally accepted set of rules set by the international union of pure and applied chemistry whereas the common names do not have any rules and are easy to remember as they do not contain any digits, suffixes and prefixes.
Complete Step By Step Answer:
As per the question, the given compound is an alkene and IUPAC rules for naming alkene and cycloalkene are as follows:
1. The name should contain an ene suffix i.e., the IUPAC name ending with “ene” represents alkene or cycloalkene.
2. The longest chain chosen for the base or root must contain both carbon atoms of the double bond.
3. The chain must be numbered in such a way that the double bond gets minimum allocation i.e., it must be numbered from the end nearest the doubly bonded carbon atom.
4. If in case, more than one double bond is present in the compound then the compound is named as diene, triene and so on.
5. The names of functional groups are always written in alphabetical order.
According to these rules, the numbering for the given compound will be as follows:

In the compound, an ethyl group is present at the second position and at third position, a methyl group is present. Hence, the IUPAC name of the given compound is 2-Ethyl-3-methylbut-1-ene.
Thus, option (B) is the correct answer.
Note:
Remember that we can recognize organic compounds from their common names as well. The IUPAC names strictly follow an internationally accepted set of rules set by the international union of pure and applied chemistry whereas the common names do not have any rules and are easy to remember as they do not contain any digits, suffixes and prefixes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




