
The Lewis structure of compound $COC{l_2}$ contains how many lone pairs of electrons?
Answer
558.3k+ views
Hint:First we have to know what is Lewis structure, how to draw the Lewis structure and then draw the structure to find out the lone pairs (the electron pairs which are not bonding with any other elements) on the central atom.
Complete answer:
Firstly starting with the definition of the Lewis structure:
Lewis structure: The representation of the valence shell electrons of a molecule in the easiest way. In these types of structures it is easy to know how the different electrons are arranged around each element in the molecules. Each electron are represented by dots that’s why it is also known as the Lewis dot structure.
Now, we are talking about how to draw the Lewis structure:
So, the Lewis dot structure is given:
First write all the elements (The elements which are bonded to each other are suggested to be drawn adjacent to each other. So, it's easy to draw the structures. ). Now, draw the valence electrons around the atoms, each electron each dot (better to draw the lone pairs to be in pairs. So, if we have to find the number of lone pairs it would be easy.) . Now, the two atoms which are bonded to each other draw the shared electron in between them and the structure is ready.
Now, drawing the structure of the $COC{l_2}$ to make it clearer:
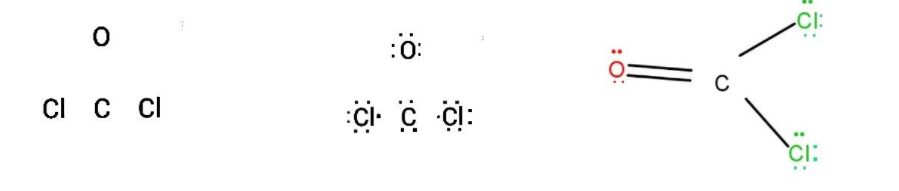
Hence, the Lewis structure of the compound $COC{l_2}$ contains an $8$ lone pair of electrons.
Note:
While drawing the Lewis dot structure of the compounds, the valence electrons of all the elements taking part in the compound should be known. A bond is represented as two dots between the two bonding elements, as the two electrons are shared between them.
Complete answer:
Firstly starting with the definition of the Lewis structure:
Lewis structure: The representation of the valence shell electrons of a molecule in the easiest way. In these types of structures it is easy to know how the different electrons are arranged around each element in the molecules. Each electron are represented by dots that’s why it is also known as the Lewis dot structure.
Now, we are talking about how to draw the Lewis structure:
So, the Lewis dot structure is given:
First write all the elements (The elements which are bonded to each other are suggested to be drawn adjacent to each other. So, it's easy to draw the structures. ). Now, draw the valence electrons around the atoms, each electron each dot (better to draw the lone pairs to be in pairs. So, if we have to find the number of lone pairs it would be easy.) . Now, the two atoms which are bonded to each other draw the shared electron in between them and the structure is ready.
Now, drawing the structure of the $COC{l_2}$ to make it clearer:
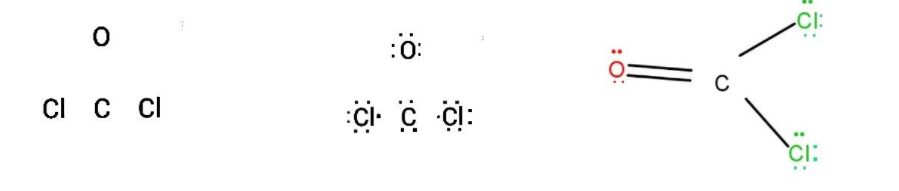
Hence, the Lewis structure of the compound $COC{l_2}$ contains an $8$ lone pair of electrons.
Note:
While drawing the Lewis dot structure of the compounds, the valence electrons of all the elements taking part in the compound should be known. A bond is represented as two dots between the two bonding elements, as the two electrons are shared between them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




