
The major product of given reactions is?
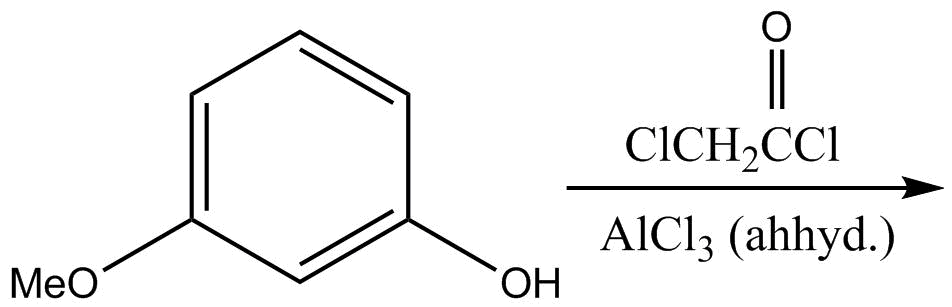
Options are:
A.
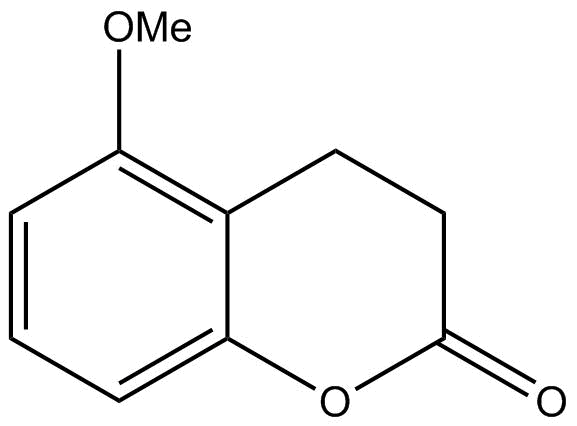
B.
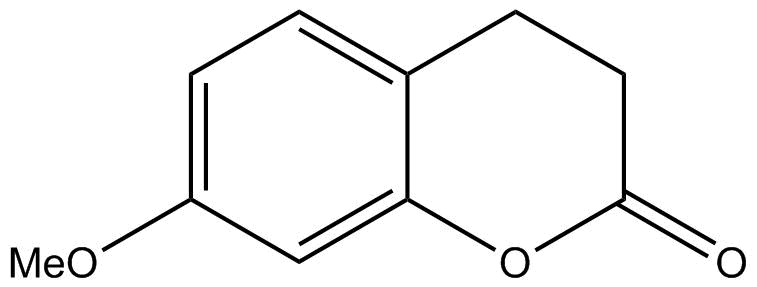
C.
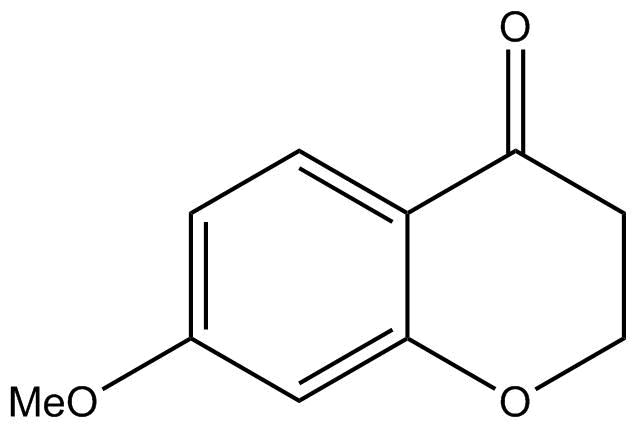
D.
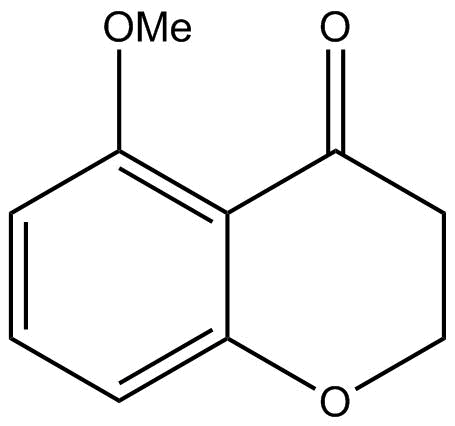





Answer
572.1k+ views
Hint: To find the major products we need to find what things are reacted with compound to form the resulting product. If we two compounds are given that means two steps are done on a single compound. Also we need to find the type of reaction happening in each step.
Complete step by step solution:
So if we see the given reaction, two things are reacted with the given compound.
In the first step $ClCH_2CH_2C = OCl$ is reacted with given compound and following result will be there:
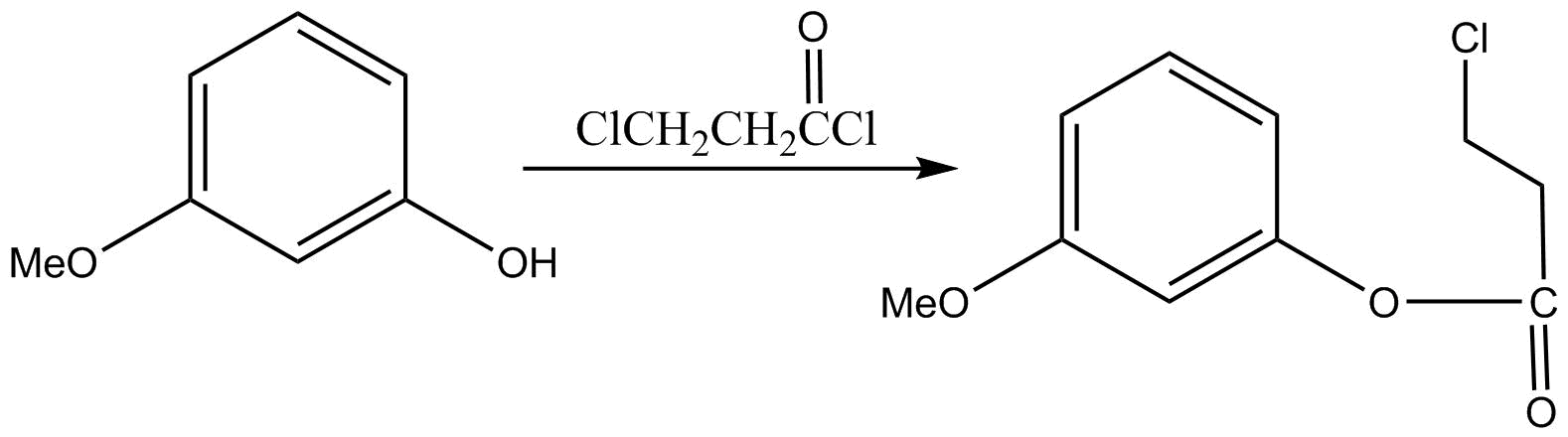
We can see acid chloride is more reactive than alkyl halide. That’s why the $ - COCl$ group will react first forming the above reaction.
Now in the second step, the product produced from above reaction will be reacted with \[AlC{l_3}\] and the following result will be there:
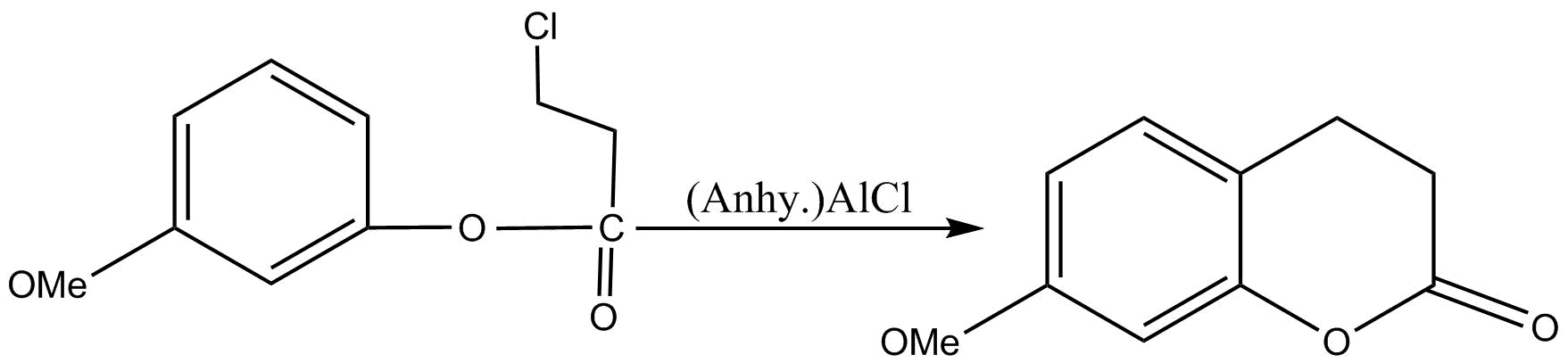
So in this step, Friedel Crafts alkylation will occur in a position such that ortho to alkoxy group and para to methoxy group will be replaced. Now both methoxy as well as alkoxy groups are ortho para directing groups.
The product formed by doing above two steps will be the major product. Hence option B is correct.
Additional Information:
Acid chloride is more reactive than alkyl halide. This is due to the reason for electronegative groups such as chlorine which polarize the carbonyl group more strongly than the alkyl group. That is why the acid chloride will react first in any reaction.
Friedel Crafts reactions are given by Charles Friedel and James Crafts. In these reactions they have a set of reactions in which they attach substitutes to the aromatic rings. Basically they are of two types: alkylation reactions and acylation reactions. Both these work with electrophilic aromatic substitution.
Friedel Crafts alkylation which is used in the above question involves alkylation of aromatic rings with alkyl halide using strong acid such as aluminum chloride.
Another type of Friedel Crafts reaction is Friedel Crafts acylation which involves acylation of aromatic rings. Acylation agents are acyl chlorides.
Note: To solve the questions of finding products we should focus on the type of reaction happening. Also we should check how many steps are done to find major products. One main point to be remembered is acid chloride is strongly reactive and if aluminum chloride is involved in the reaction it is Friedel crafts reaction.
Complete step by step solution:
So if we see the given reaction, two things are reacted with the given compound.
In the first step $ClCH_2CH_2C = OCl$ is reacted with given compound and following result will be there:

We can see acid chloride is more reactive than alkyl halide. That’s why the $ - COCl$ group will react first forming the above reaction.
Now in the second step, the product produced from above reaction will be reacted with \[AlC{l_3}\] and the following result will be there:

So in this step, Friedel Crafts alkylation will occur in a position such that ortho to alkoxy group and para to methoxy group will be replaced. Now both methoxy as well as alkoxy groups are ortho para directing groups.
The product formed by doing above two steps will be the major product. Hence option B is correct.
Additional Information:
Acid chloride is more reactive than alkyl halide. This is due to the reason for electronegative groups such as chlorine which polarize the carbonyl group more strongly than the alkyl group. That is why the acid chloride will react first in any reaction.
Friedel Crafts reactions are given by Charles Friedel and James Crafts. In these reactions they have a set of reactions in which they attach substitutes to the aromatic rings. Basically they are of two types: alkylation reactions and acylation reactions. Both these work with electrophilic aromatic substitution.
Friedel Crafts alkylation which is used in the above question involves alkylation of aromatic rings with alkyl halide using strong acid such as aluminum chloride.
Another type of Friedel Crafts reaction is Friedel Crafts acylation which involves acylation of aromatic rings. Acylation agents are acyl chlorides.
Note: To solve the questions of finding products we should focus on the type of reaction happening. Also we should check how many steps are done to find major products. One main point to be remembered is acid chloride is strongly reactive and if aluminum chloride is involved in the reaction it is Friedel crafts reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




