
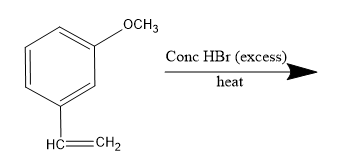
The major product of the following reactions:
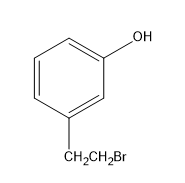
(A)
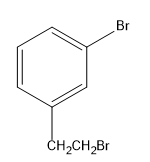
(B)
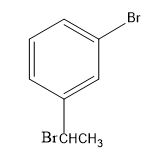
(C)
(D)
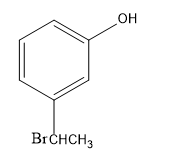





Answer
577.8k+ views
Hint: In the presence of the protic acid (HBr), the hydrohalogenation reaction will occur, through the electrophilic addition reaction mechanism. This leads to the protonation of the alkene.
Complete step by step answer:
The given compound is 3-methoxy ethenylbenzene or m-methoxy styrene which on reaction with concentrated hydrogen bromide, undergoes electrophilic addition reaction following Markovnikov's rule. Thus, it is an hydrohalogenation reaction of the unsymmetrical alkene.
-Form the Markovnikov’s rule the addition of the hydrogen bromide to the alkene is such that the proton gets added to the carbon with more hydrogen substituents and the bromide ion gets attached to the carbon with more alkyl substituents.
-So, in the reaction, the ${{H}^{+}}$ from the hydrogen bromide acts as an electrophile and is attached to the terminal carbon with more hydrogen substituents. This is followed by the formation of carbocation on the carbon with more alkyl substituent. As the benzyl carbocation formed is more stable than the alkyl carbocation.
-Then, the bromide gets attached to the carbocation, forming bromo-substituted alkane, that is, m-(1-bromoethyl) anisole.
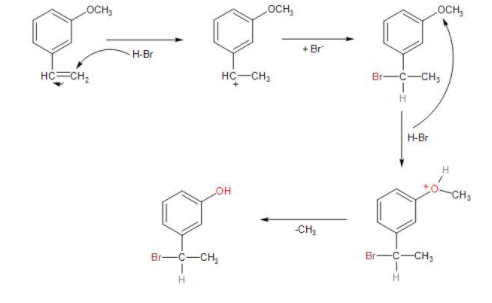
As the protic acid is present in excess, it causes further reaction to occur with the methoxy group. As the proton as electrophile attacks the oxygen atom of the methoxy group, the methyl group being a better leaving group departs. Thus, forming m-(1-bromoethyl) phenol.
Therefore, the major product of the given reaction is option (D) m-(1-bromoethyl) phenol.
Note: The Markovnikov’s rule is applied here due to the absence of the benzoyl peroxide, otherwise the Anti-Markovnikov’s rule would have been applicable.
In Markovnikov's rule, the proton in the protic acid acts as the electrophile and the stability of the carbocation formed is given preference.
Complete step by step answer:
The given compound is 3-methoxy ethenylbenzene or m-methoxy styrene which on reaction with concentrated hydrogen bromide, undergoes electrophilic addition reaction following Markovnikov's rule. Thus, it is an hydrohalogenation reaction of the unsymmetrical alkene.
-Form the Markovnikov’s rule the addition of the hydrogen bromide to the alkene is such that the proton gets added to the carbon with more hydrogen substituents and the bromide ion gets attached to the carbon with more alkyl substituents.
-So, in the reaction, the ${{H}^{+}}$ from the hydrogen bromide acts as an electrophile and is attached to the terminal carbon with more hydrogen substituents. This is followed by the formation of carbocation on the carbon with more alkyl substituent. As the benzyl carbocation formed is more stable than the alkyl carbocation.
-Then, the bromide gets attached to the carbocation, forming bromo-substituted alkane, that is, m-(1-bromoethyl) anisole.

As the protic acid is present in excess, it causes further reaction to occur with the methoxy group. As the proton as electrophile attacks the oxygen atom of the methoxy group, the methyl group being a better leaving group departs. Thus, forming m-(1-bromoethyl) phenol.
Therefore, the major product of the given reaction is option (D) m-(1-bromoethyl) phenol.
Note: The Markovnikov’s rule is applied here due to the absence of the benzoyl peroxide, otherwise the Anti-Markovnikov’s rule would have been applicable.
In Markovnikov's rule, the proton in the protic acid acts as the electrophile and the stability of the carbocation formed is given preference.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




