
The major product of the given reaction is:
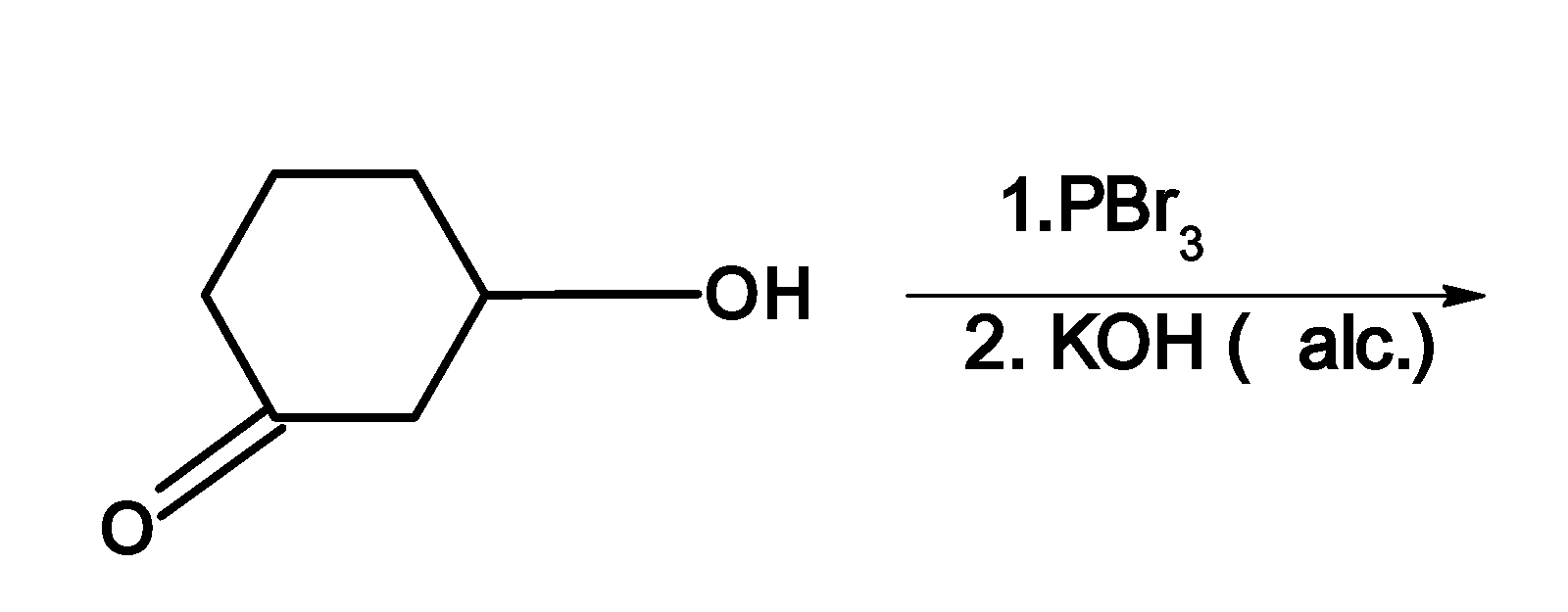
A)
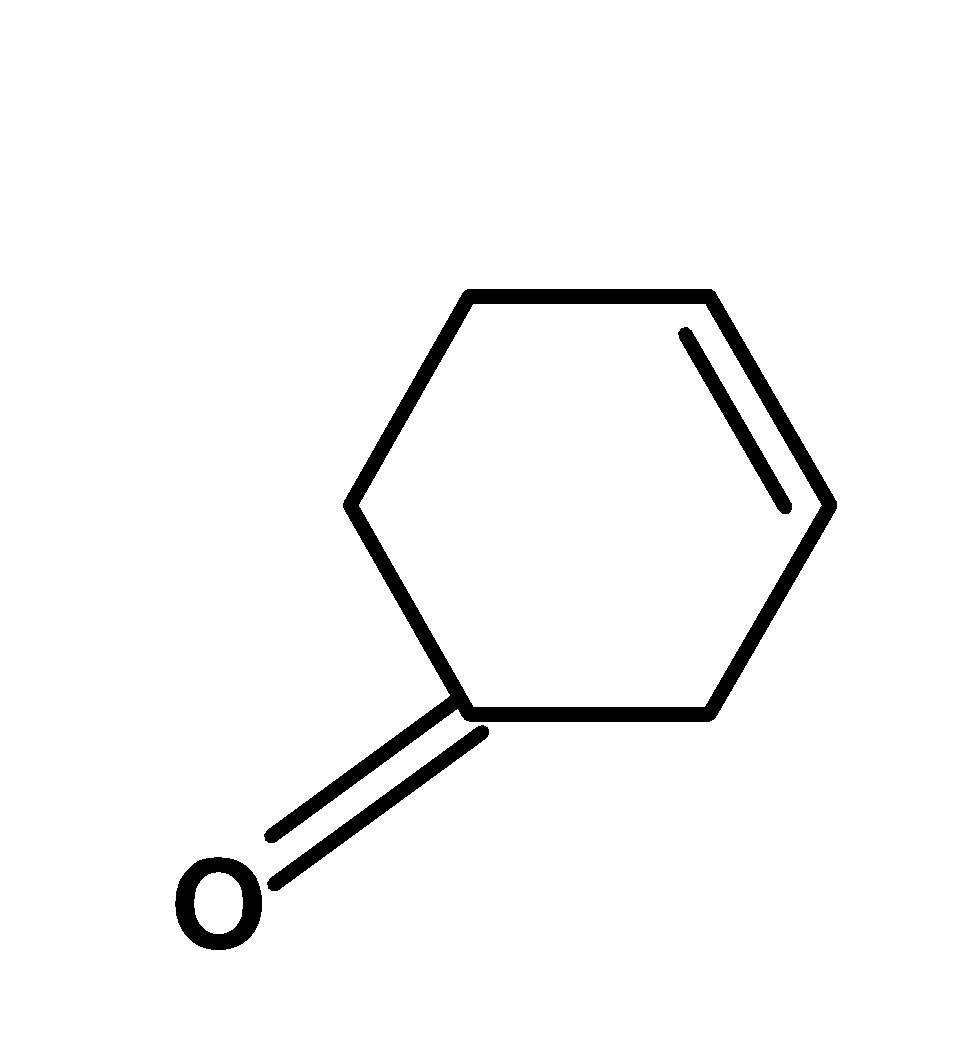
B)
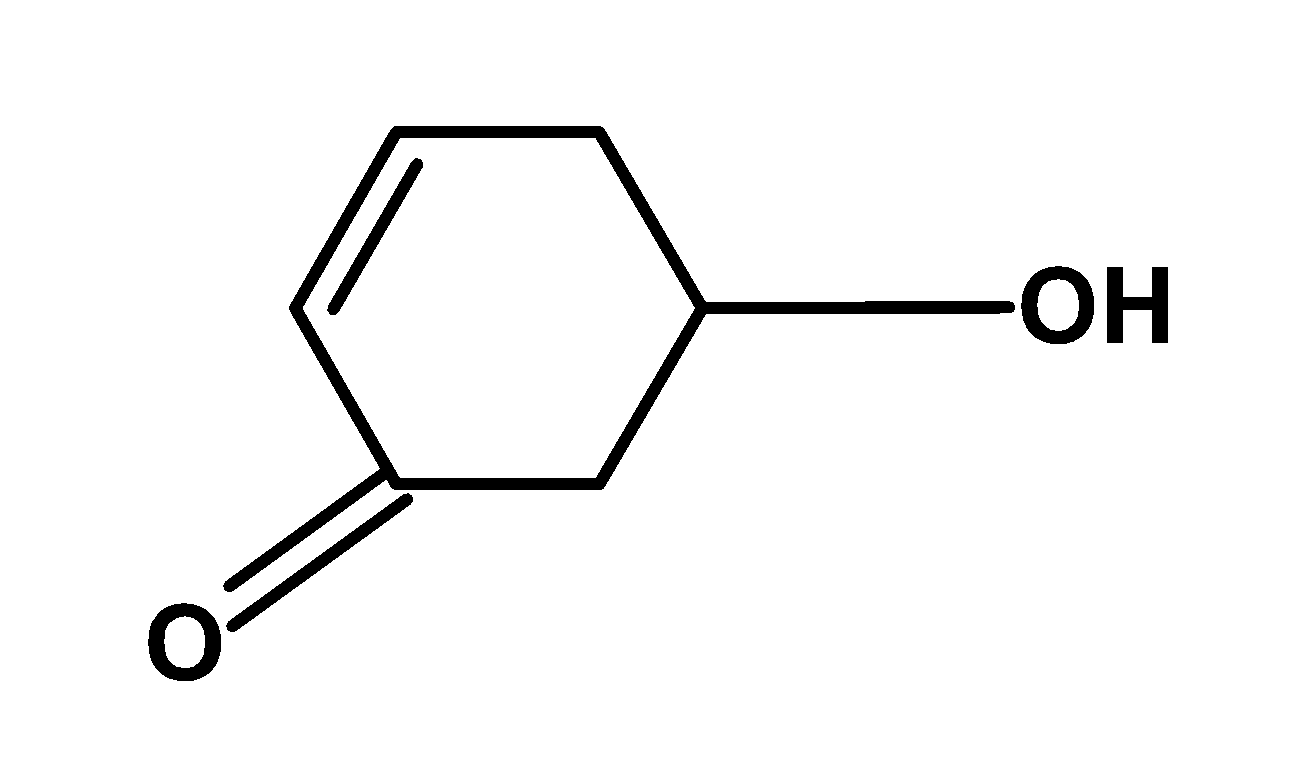
C)

D)


| A) | 
|
| B) | 
|
| C) | 
|
| D) | 
|
Answer
579k+ views
Hint: The alcohol reacts with the phosphorus halide (like $\text{ PB}{{\text{r}}_{\text{3}}}\text{ }$or $\text{ PC}{{\text{l}}_{\text{3}}}\text{ }$).This reagent act as halogen rich species. The reaction of phosphorus halide with the alcohol produces alkyl halide. The alkyl halide when treated with the alcoholic potassium hydroxide produced the $\text{ }\beta \text{ }$elimination product.
Complete step by step answer:
-Phosphorus halides like phosphorus tribromide $\text{ PB}{{\text{r}}_{\text{3}}}\text{ }$ or $\text{ PC}{{\text{l}}_{\text{3}}}\text{ }$ can donate or accept the electrons thus act as the Lewis acid or Lewis base.
-One of the most important reactions of phosphorus bromide is with alcohol. It is a bromide rich reagent. Thus it donates its bromide ions to the alcohol. The hydroxyl group $\text{ }-\text{OH }$ of the alcohol is replaced by the bromine atom and produces alkyl halide having formula $\text{ R}-\text{Br }$ .
-The phosphorus bromide transferred its three bromides to the three alcohol molecules. The general reaction is as shown below,
$\text{ 3 ROH + PB}{{\text{r}}_{\text{3}}}\text{ }\to \text{ 3RBr + HP(O)(OH}{{\text{)}}_{\text{2}}}\text{ }$
-The reaction follows the nucleophilic substitution bimolecular $\text{ }{{\text{S}}_{\text{N}}}\text{2 }$ reaction. The oxygen atom in the alcohol acts as the nucleophile. It attacks the phosphorus followed by the removal of bromide ion.
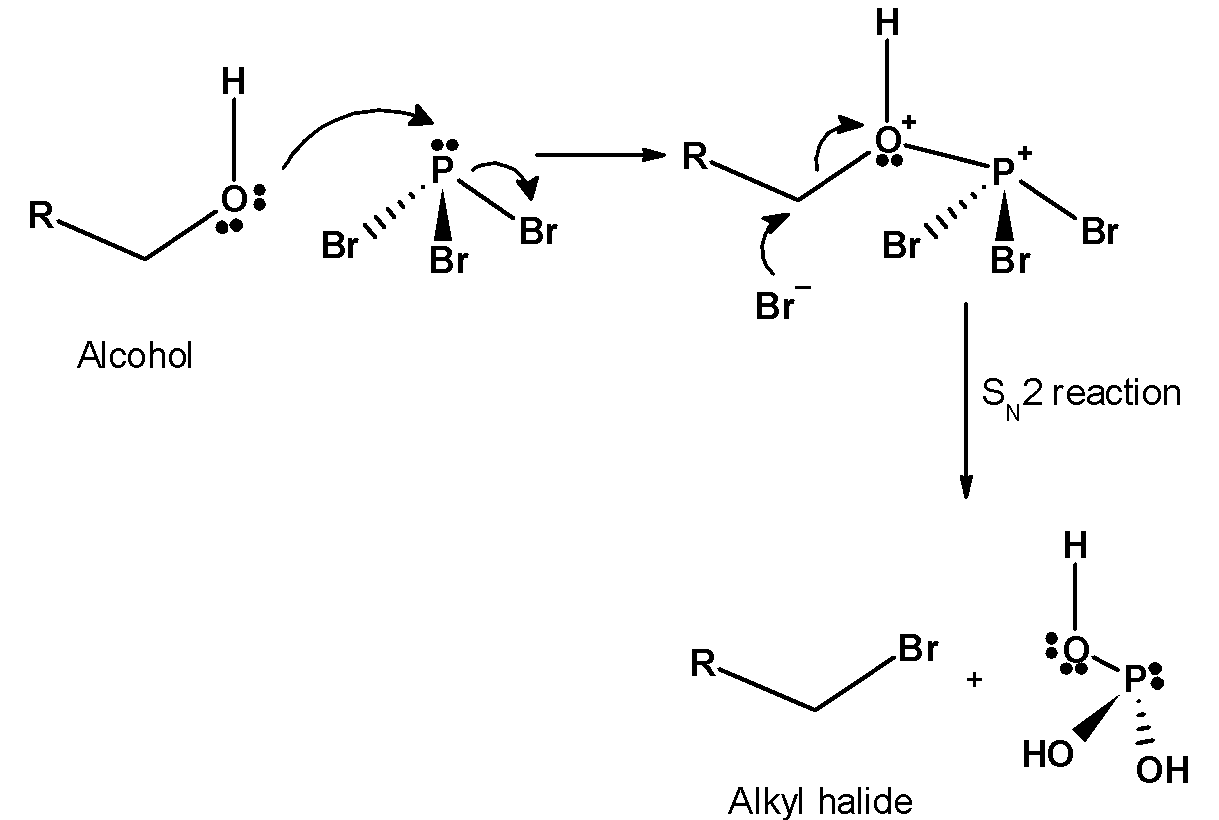
-It is a single step mechanism. In the next step, the bromide ion (earlier leaving group) attacks on the carbon atom adjacent to the oxygen atom with the removal of $\text{ P (Br}{{\text{)}}_{\text{2}}}\text{OH }$.this further attacks on the two alcohol molecules.
-Thus, the given compound reacts with the $\text{ PB}{{\text{r}}_{\text{3}}}\text{ }$and forms the halide as follows,
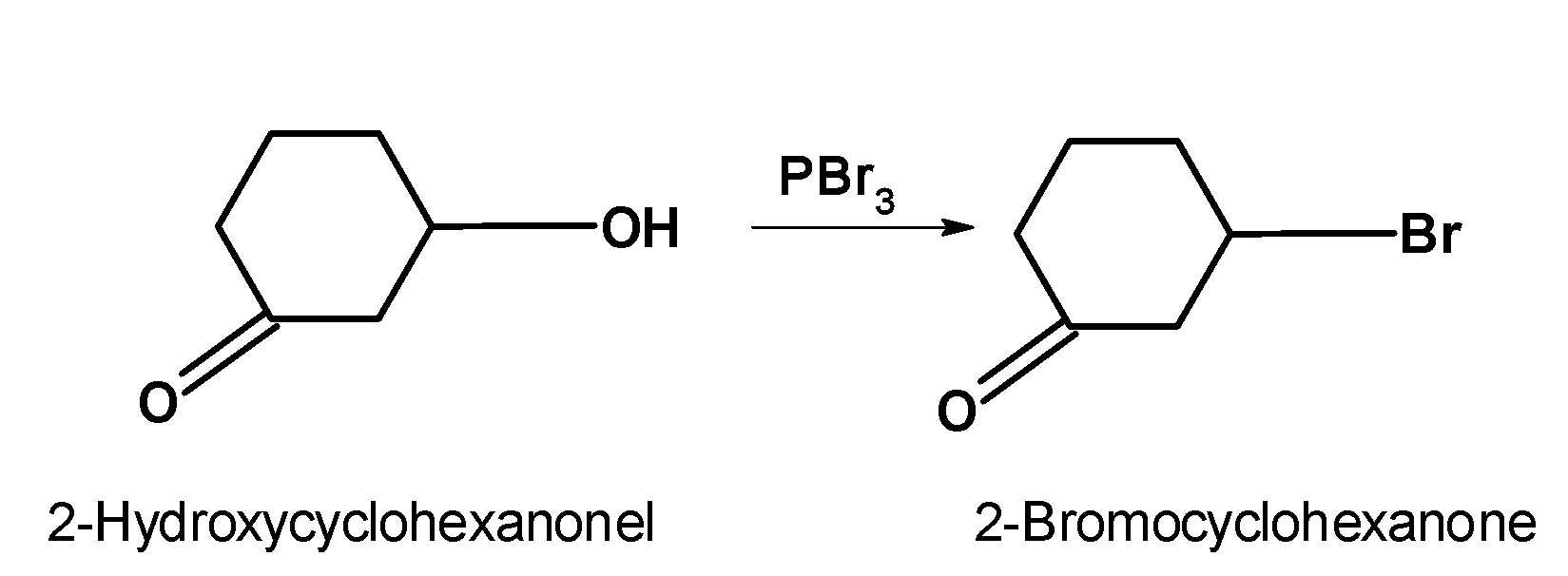
-When haloalkanes with $\text{ }\beta \text{ }$ hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo an elimination reaction of hydrogen halide $\text{ HX }$ resulting in the formation of alkenes. These reactions are called as the $\text{ }\beta \text{ }$elimination reaction because hydrogen present at the $\text{ }\beta \text{ }$position of the haloalkanes is removed.
-The obtained product i.e. 2-bromocyclohexanone is treated with the alcoholic and we get,
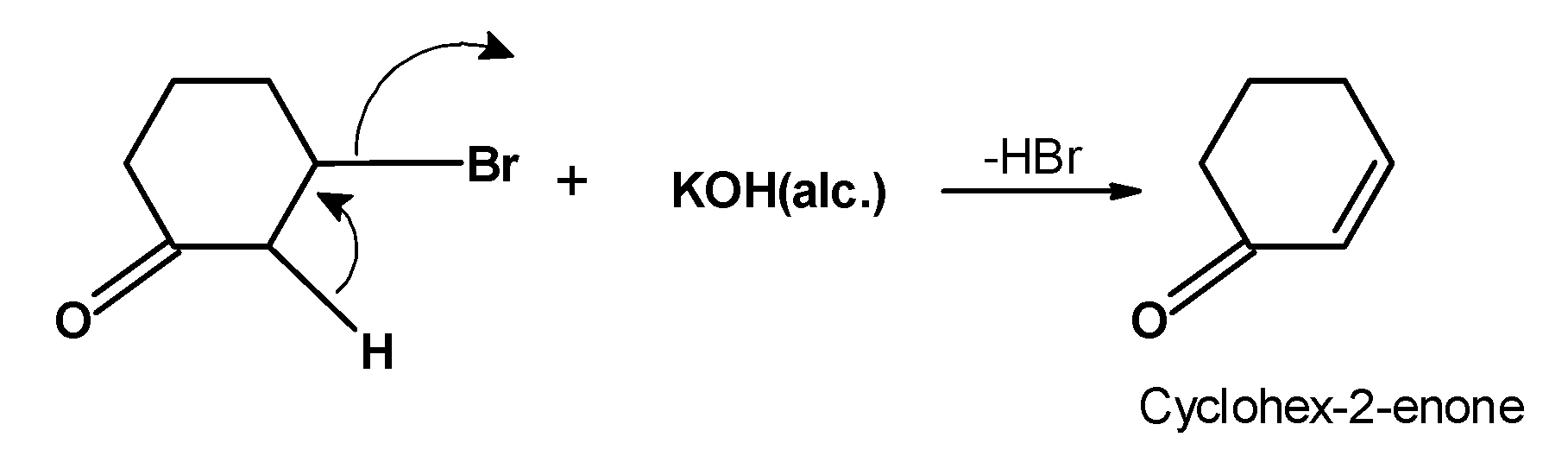
-There is one more possibility of the product when the double bond is on another beta hydrogen atom. However, the carbonyl group attracts the electron density, and thus instead of cyclohexane-3-none, the major product is cyclohex-2-enone.
Hence, (D) is the correct option.
Note: The reaction of phosphorus bromide works well with primary and secondary alcohol. This is because the substitution bimolecular reaction is more favourable in absence of crowding. The reaction of haloalkanes with the potassium hydroxide (alcoholic) is also known as the dehydrohalogenation reaction, as the reaction involves the removal of hydrogen halide.
Complete step by step answer:
-Phosphorus halides like phosphorus tribromide $\text{ PB}{{\text{r}}_{\text{3}}}\text{ }$ or $\text{ PC}{{\text{l}}_{\text{3}}}\text{ }$ can donate or accept the electrons thus act as the Lewis acid or Lewis base.
-One of the most important reactions of phosphorus bromide is with alcohol. It is a bromide rich reagent. Thus it donates its bromide ions to the alcohol. The hydroxyl group $\text{ }-\text{OH }$ of the alcohol is replaced by the bromine atom and produces alkyl halide having formula $\text{ R}-\text{Br }$ .
-The phosphorus bromide transferred its three bromides to the three alcohol molecules. The general reaction is as shown below,
$\text{ 3 ROH + PB}{{\text{r}}_{\text{3}}}\text{ }\to \text{ 3RBr + HP(O)(OH}{{\text{)}}_{\text{2}}}\text{ }$
-The reaction follows the nucleophilic substitution bimolecular $\text{ }{{\text{S}}_{\text{N}}}\text{2 }$ reaction. The oxygen atom in the alcohol acts as the nucleophile. It attacks the phosphorus followed by the removal of bromide ion.

-It is a single step mechanism. In the next step, the bromide ion (earlier leaving group) attacks on the carbon atom adjacent to the oxygen atom with the removal of $\text{ P (Br}{{\text{)}}_{\text{2}}}\text{OH }$.this further attacks on the two alcohol molecules.
-Thus, the given compound reacts with the $\text{ PB}{{\text{r}}_{\text{3}}}\text{ }$and forms the halide as follows,

-When haloalkanes with $\text{ }\beta \text{ }$ hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo an elimination reaction of hydrogen halide $\text{ HX }$ resulting in the formation of alkenes. These reactions are called as the $\text{ }\beta \text{ }$elimination reaction because hydrogen present at the $\text{ }\beta \text{ }$position of the haloalkanes is removed.
-The obtained product i.e. 2-bromocyclohexanone is treated with the alcoholic and we get,
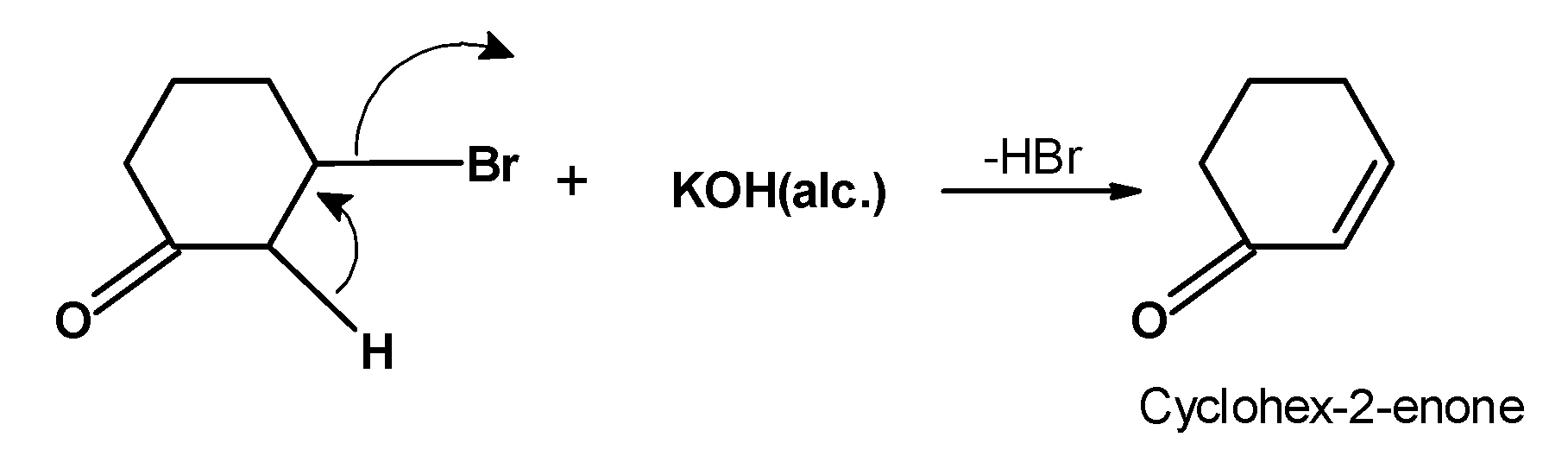
-There is one more possibility of the product when the double bond is on another beta hydrogen atom. However, the carbonyl group attracts the electron density, and thus instead of cyclohexane-3-none, the major product is cyclohex-2-enone.
Hence, (D) is the correct option.
Note: The reaction of phosphorus bromide works well with primary and secondary alcohol. This is because the substitution bimolecular reaction is more favourable in absence of crowding. The reaction of haloalkanes with the potassium hydroxide (alcoholic) is also known as the dehydrohalogenation reaction, as the reaction involves the removal of hydrogen halide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




