
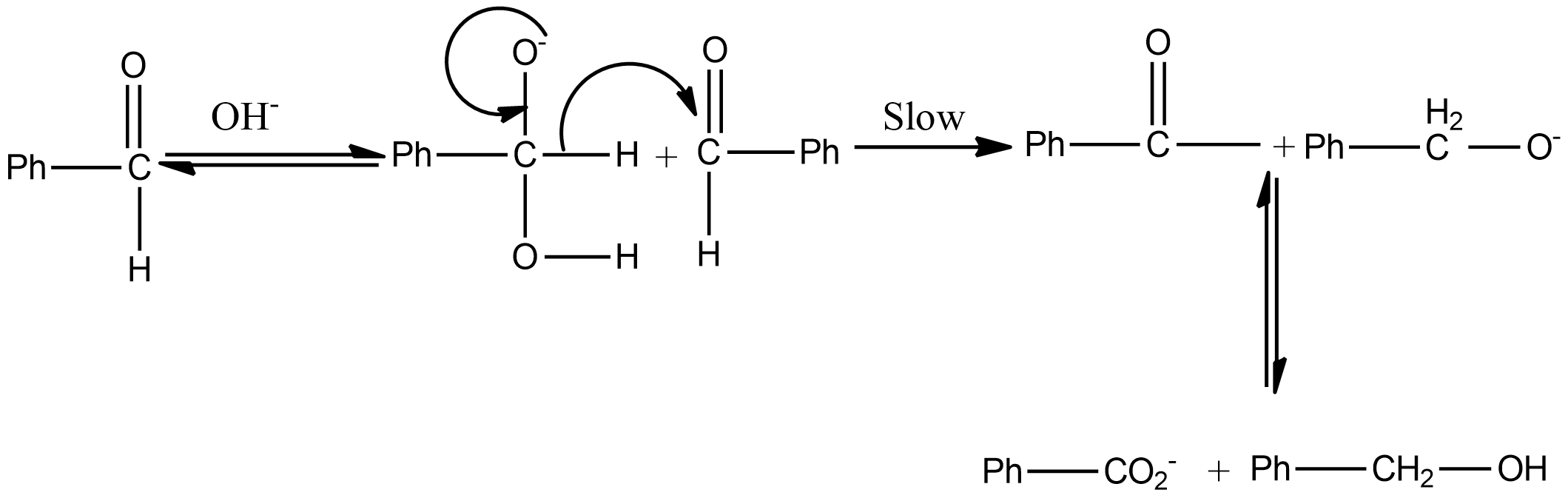
The mechanism of Cannizzaro's reaction of benzaldehyde is
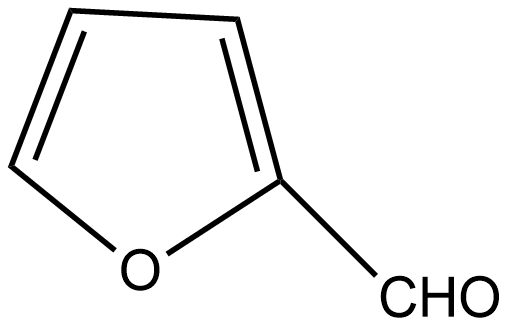
 Which of the following reactants can undergo Cannizzaro's reaction?
Which of the following reactants can undergo Cannizzaro's reaction?
A.

B. $R_3CCHO$
C.
D. All of these



Answer
568.8k+ views
Hint: Cannizzaro reaction is a disproportionation reaction that means the reaction involves the self oxidation and reduction reaction. As a result of the reaction, one molecule of the reactant was reduced to alcohol and the other oxidized to the carboxylic acid.
Complete step by step answer:
As we know that the aldehyde which does not contain α hydrogen undergoes the Cannizzaro reaction. If we talk about option A i.e. formaldehyde or methanal, there is no $\alpha$ hydrogen thus it undergoes the Cannizzaro reaction.
If we talk about the in option B, there is a presence of α hydrogen thus it does not undergo a Cannizzaro reaction.
If we talk about option C, there is also no α hydrogen thus it undergoes the Cannizzaro reaction.
So, the correct answer is Option A,C .
Additional information:
If two different aldehydes are present as a reactant then the reaction carried out is known as the cross Cannizzaro reaction. If one of the reactants is formaldehyde then the cross Cannizzaro reaction is of a great synthetic utility and it gives sodium formate and alcohol corresponding to the other aldehyde.
Note: It may be noted that the Cannizzaro reaction is the characteristic of aldehydes having no α hydrogen, but it is not confirmed to them. Certain aliphatic monoalkylated aldehydes undergo disproportionation reactions when heated with aq. NaOH. This exceptional behavior is probably due to the +I effect of two alkyl groups. In this aldehydes prefer to undergo a nucleophilic attack by $O{H^ - }$ ion at the aldehyde group giving intermediate which acts as a hydride donor to the second molecule of aldehyde to give disproportionation reaction.
Complete step by step answer:
As we know that the aldehyde which does not contain α hydrogen undergoes the Cannizzaro reaction. If we talk about option A i.e. formaldehyde or methanal, there is no $\alpha$ hydrogen thus it undergoes the Cannizzaro reaction.
If we talk about the in option B, there is a presence of α hydrogen thus it does not undergo a Cannizzaro reaction.
If we talk about option C, there is also no α hydrogen thus it undergoes the Cannizzaro reaction.
So, the correct answer is Option A,C .
Additional information:
If two different aldehydes are present as a reactant then the reaction carried out is known as the cross Cannizzaro reaction. If one of the reactants is formaldehyde then the cross Cannizzaro reaction is of a great synthetic utility and it gives sodium formate and alcohol corresponding to the other aldehyde.
Note: It may be noted that the Cannizzaro reaction is the characteristic of aldehydes having no α hydrogen, but it is not confirmed to them. Certain aliphatic monoalkylated aldehydes undergo disproportionation reactions when heated with aq. NaOH. This exceptional behavior is probably due to the +I effect of two alkyl groups. In this aldehydes prefer to undergo a nucleophilic attack by $O{H^ - }$ ion at the aldehyde group giving intermediate which acts as a hydride donor to the second molecule of aldehyde to give disproportionation reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




