
The molecular formula of dithionic acid is:
A.${H_2}{S_2}{O_4}$
B.${H_2}{S_2}{O_6}$
C.${H_2}{S_2}{O_5}$
D.${H_2}{S_2}{O_7}$
Answer
569.4k+ views
Hint:
Dithionic acid is the oxoacid of sulphur. Oxoacids are those acids which are identified by the presence of oxygen atoms in an acid. In oxoacid oxygen is bonded to hydrogen atom. There are different types of oxoacids of sulphur that exist.
Complete answer:
The structure of dithionic acid is given below as follows:
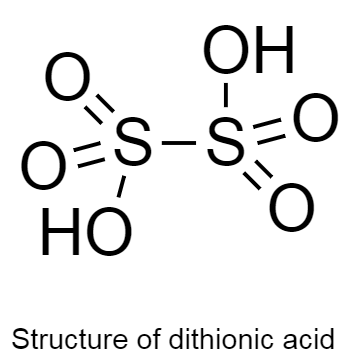
In the given structure there are two hydrogen atoms bonded to two oxygen atoms.
There are two hydrogen, six oxygen and two sulphur atoms in the structure.
From this we can say that the molecular formula of dithionic acid is ${H_2}{S_2}{O_6}$ .
The oxidation state of sulphur in dithionic acid is $ + 5$ .
The oxoacids of sulphur are tetrahedral in structure when they are bonded to oxygen atoms.
These oxoacids contain at least one sulphur-oxygen double bond and one sulphur-hydroxide bond.
So, the correct answer is option b) ${H_2}{S_2}{O_6}$
Additional information:
Some examples of oxoacids of sulphur are namely: sulphuric acid${H_2}S{O_4}$ , Sulfurous acid ${H_2}S{O_4}$ , peroxodisulfuric acid ${H_2}{S_2}{O_8}$ .
Thionic acids are unstable compared to other oxoacids of sulphur.
Oxyacids dissociate in water to form acid anion and hydrogen cations.
These oxyacids are only important for the salts that are obtained from them.
Otherwise they do not have any other application.
Sulphuric acid is the only oxoacid that is used widely in industries for numerous purposes.
Note:The electronegativity of the central metal atom and the total number of oxygen atoms are responsible for the acidic character in oxoacids.
The hydrogen that is bonded to oxygen in oxyacids is acidic in nature.
Dithionic acid is the oxoacid of sulphur. Oxoacids are those acids which are identified by the presence of oxygen atoms in an acid. In oxoacid oxygen is bonded to hydrogen atom. There are different types of oxoacids of sulphur that exist.
Complete answer:
The structure of dithionic acid is given below as follows:

In the given structure there are two hydrogen atoms bonded to two oxygen atoms.
There are two hydrogen, six oxygen and two sulphur atoms in the structure.
From this we can say that the molecular formula of dithionic acid is ${H_2}{S_2}{O_6}$ .
The oxidation state of sulphur in dithionic acid is $ + 5$ .
The oxoacids of sulphur are tetrahedral in structure when they are bonded to oxygen atoms.
These oxoacids contain at least one sulphur-oxygen double bond and one sulphur-hydroxide bond.
So, the correct answer is option b) ${H_2}{S_2}{O_6}$
Additional information:
Some examples of oxoacids of sulphur are namely: sulphuric acid${H_2}S{O_4}$ , Sulfurous acid ${H_2}S{O_4}$ , peroxodisulfuric acid ${H_2}{S_2}{O_8}$ .
Thionic acids are unstable compared to other oxoacids of sulphur.
Oxyacids dissociate in water to form acid anion and hydrogen cations.
These oxyacids are only important for the salts that are obtained from them.
Otherwise they do not have any other application.
Sulphuric acid is the only oxoacid that is used widely in industries for numerous purposes.
Note:The electronegativity of the central metal atom and the total number of oxygen atoms are responsible for the acidic character in oxoacids.
The hydrogen that is bonded to oxygen in oxyacids is acidic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




