
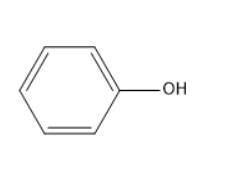
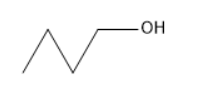
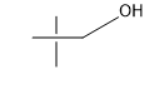
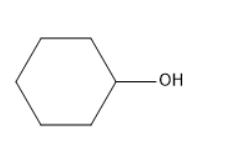
The molecule which does not oxidize using PCC?
(A)
(B)
(C)
(D)




Answer
480.3k+ views
Hint: Alcohols are chemical compounds consisting of hydroxyl groups. Oxidation of alcohols by strong oxidizing agents gives carboxylic acids, while oxidizing agents give carbonyl compounds only. PCC is a weak oxidizing agent and reduces the primary alcohols to aldehydes and secondary alcohols to ketones.
Complete answer:
Reagents were classified into different types based on the different classifications. One of the classifications is oxidizing and reducing agents. Oxidizing agents are useful for the oxidation of other substances whereas they undergo reduction. Similarly reducing agents, themselves undergo oxidation and reduce the other substances.
Oxidizing agents were classified into two types. They are strong and weak oxidizing agents. PCC is known as pyridinium chlorochromate. It is a weak oxidizing agent.
PCC can oxidize the compounds which contain hydrogen atoms attached to the carbon atom adjacent to oxygen in the hydroxyl group.
The structure of PCC is:

Option A does not consist of hydrogen atoms attached to carbon adjacent to oxygen of hydroxyl group. Thus, it is the correct answer.
In the options B, C, and D there was a hydrogen atom attached to the carbon adjacent to the oxygen of the hydroxyl group. Thus, these are not the correct answers.
Option A is nothing but phenol, which cannot be oxidized by using PCC.
Option A is the correct one.
Note:
As PCC is a weak oxidizing agent, it cannot oxidize the primary alcohols directly to carboxylic acids but oxidizes primary alcohols to aldehydes only. It can oxidize only primary and secondary alcohols but not tertiary alcohols.
Complete answer:
Reagents were classified into different types based on the different classifications. One of the classifications is oxidizing and reducing agents. Oxidizing agents are useful for the oxidation of other substances whereas they undergo reduction. Similarly reducing agents, themselves undergo oxidation and reduce the other substances.
Oxidizing agents were classified into two types. They are strong and weak oxidizing agents. PCC is known as pyridinium chlorochromate. It is a weak oxidizing agent.
PCC can oxidize the compounds which contain hydrogen atoms attached to the carbon atom adjacent to oxygen in the hydroxyl group.
The structure of PCC is:

Option A does not consist of hydrogen atoms attached to carbon adjacent to oxygen of hydroxyl group. Thus, it is the correct answer.
In the options B, C, and D there was a hydrogen atom attached to the carbon adjacent to the oxygen of the hydroxyl group. Thus, these are not the correct answers.
Option A is nothing but phenol, which cannot be oxidized by using PCC.
Option A is the correct one.
Note:
As PCC is a weak oxidizing agent, it cannot oxidize the primary alcohols directly to carboxylic acids but oxidizes primary alcohols to aldehydes only. It can oxidize only primary and secondary alcohols but not tertiary alcohols.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




