
The monomer unit of PVC is:
A. Propene
B. Vinyl chloride
C. Vulcanised rubber
D. None of the above
Answer
577.5k+ views
Hint: The full name of PVC is polyvinyl chloride. PVC is a homopolymer. The name of the homopolymer represents the name of the monomer.
Step by step answer: When a large number of molecules join in a manner to form a long structure, the structure is known as a polymer. In a polymer, a molecule or unit repeats again, and again that’s why the formed structure is known as a polymer because the word ‘poly‘ means many, and ‘mer’ means units.
The units which repeat are known as repeating units or monomers.
Based on the type of polymer units the polymer is divided into two categories:
Homopolymer: When only one type of repeating unit is present in a polymer the polymer is known as a homopolymer.
Copolymer: When more than one type of repeating unit is present in a polymer the polymer is known as copolymer.
The polymer polypropene is a homopolymer. The repeating unit or monomer of polypropene is propene. So, the propene is not the monomer unit of PVC so option (A) is incorrect.
The repeating unit or monomer of PVC is vinyl chloride.
The structure of vinyl chloride is as follows:
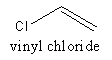
The polymer of PVC is represented as follows:
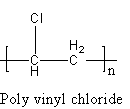
Here, n is the number of units.
The monomer unit of polymer polyvinyl chloride is vinyl chloride so, option (B) is correct.
Vulcanized rubber is a natural rubber treated with curatives for hardening. Vulcanized rubber itself is a polymer not the monomer of PVC
so option (C) is incorrect.
Note: The name of the homopolymer includes the name of the monomer unit from which the polymer is derived and the prefix ‘poly’. The polymer polyvinyl chloride and polypropene both are homopolymer.
Step by step answer: When a large number of molecules join in a manner to form a long structure, the structure is known as a polymer. In a polymer, a molecule or unit repeats again, and again that’s why the formed structure is known as a polymer because the word ‘poly‘ means many, and ‘mer’ means units.
The units which repeat are known as repeating units or monomers.
Based on the type of polymer units the polymer is divided into two categories:
Homopolymer: When only one type of repeating unit is present in a polymer the polymer is known as a homopolymer.
Copolymer: When more than one type of repeating unit is present in a polymer the polymer is known as copolymer.
The polymer polypropene is a homopolymer. The repeating unit or monomer of polypropene is propene. So, the propene is not the monomer unit of PVC so option (A) is incorrect.
The repeating unit or monomer of PVC is vinyl chloride.
The structure of vinyl chloride is as follows:

The polymer of PVC is represented as follows:

Here, n is the number of units.
The monomer unit of polymer polyvinyl chloride is vinyl chloride so, option (B) is correct.
Vulcanized rubber is a natural rubber treated with curatives for hardening. Vulcanized rubber itself is a polymer not the monomer of PVC
so option (C) is incorrect.
Note: The name of the homopolymer includes the name of the monomer unit from which the polymer is derived and the prefix ‘poly’. The polymer polyvinyl chloride and polypropene both are homopolymer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




