
The monomer used to produce orlon is:
A. $C{H_2} = CHF$
B. $C{H_2} = CC{l_2}$
C. $C{H_2} = CHCl$
D. $C{H_2} = CHCN$
Answer
560.7k+ views
Hint: First we should be aware of the monomer which is used in making the polymer fabric of orlon. Now the monomer is the small of basic integrative constituents which are reacted by the process of polymerization for the making of polymers. Here the monomer is $C{H_2} = CHCN$ .
Complete answer:
Here in the given question statement the question is regarding the terminology of the type of the substance which is formed when two or more atoms unite by the use of any force. This on a much permanent basis.
Orlon is a Strong and warm acrylic fiber. It has multiple uses but most often is used for making the sweaters and tracksuits and in many cases as the linings for many kinds of boots and gloves. Apart from that they are also used in the furnishing fabrics and carpets. It is mostly manufactured in the form of a filament, then cut into short staple lengths similar to wool hairs, and spun into yarn.
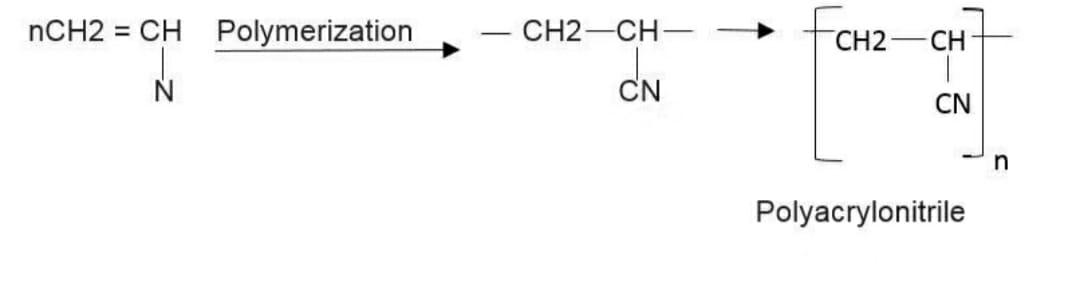
Now we know that the monomer which is used to produce orlon is acrylonitrile (vinyl cyanide) $C{H_2} = CHCN$
So we can say that Orlon is polyacrylonitrile and It is also an additional polymer.
Here is a chemical reaction showing the formation of the polymer or the process of polymerization taking place. This is done under certain circumstances and specific times.
The chemical reaction is :
 Therefore the correct option is option D, Impure metal M.
Therefore the correct option is option D, Impure metal M.
Note:Typical comonomers are vinyl acetate or methyl acrylate. DuPont created the first acrylic fibers in \[1941\] and trademarked them under the name Orlon. It was first developed in the mid \[1940s\] but was not produced in large quantities until the \[1950s\] .
Complete answer:
Here in the given question statement the question is regarding the terminology of the type of the substance which is formed when two or more atoms unite by the use of any force. This on a much permanent basis.
Orlon is a Strong and warm acrylic fiber. It has multiple uses but most often is used for making the sweaters and tracksuits and in many cases as the linings for many kinds of boots and gloves. Apart from that they are also used in the furnishing fabrics and carpets. It is mostly manufactured in the form of a filament, then cut into short staple lengths similar to wool hairs, and spun into yarn.
Now we know that the monomer which is used to produce orlon is acrylonitrile (vinyl cyanide) $C{H_2} = CHCN$
So we can say that Orlon is polyacrylonitrile and It is also an additional polymer.
Here is a chemical reaction showing the formation of the polymer or the process of polymerization taking place. This is done under certain circumstances and specific times.
The chemical reaction is :

Note:Typical comonomers are vinyl acetate or methyl acrylate. DuPont created the first acrylic fibers in \[1941\] and trademarked them under the name Orlon. It was first developed in the mid \[1940s\] but was not produced in large quantities until the \[1950s\] .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




