
The monomers of Buna-S rubber are:
A.Vinyl chloride and sulphur
B.Butadiene
C.Styrene and butadiene
D.Isoprene and butadiene
Answer
590.7k+ views
Hint: Buna-S is a synthetic polymer made up from the combination of two monomer units. It is formed in the polymerization process at low temperature that’s why it is also called cold rubber. This rubber is also termed as styrene butadiene rubber (SBR).
Complete step by step answer:
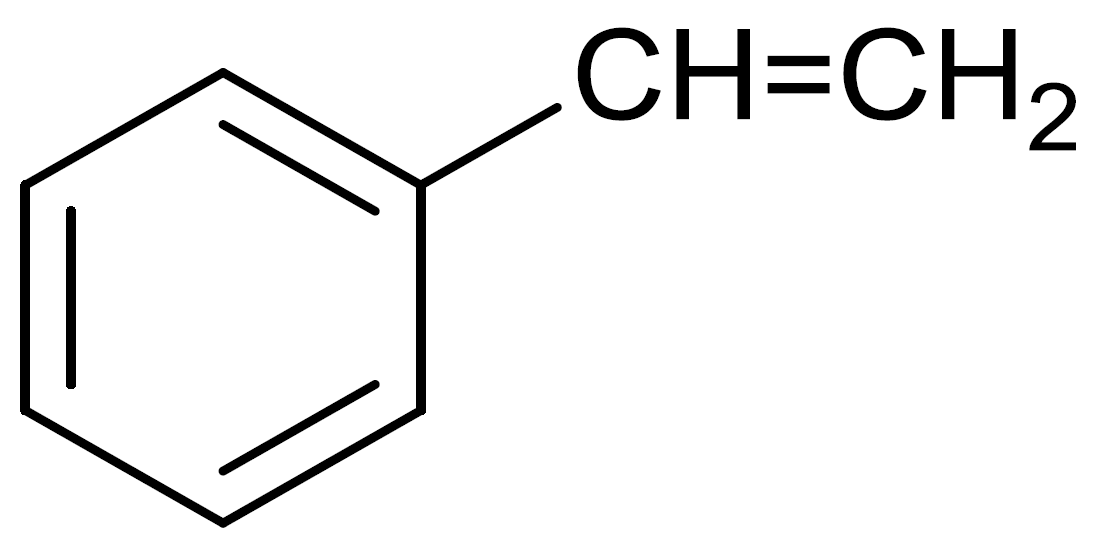
Buna-S is a synthetic polymer made up from the combination of butadiene and styrene. It is a copolymer of $75\% $ butadiene and $25\% $ styrene.
Butadiene
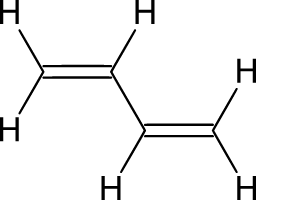
Styrene
The polymerization reaction involved in the formation of Buna-S:
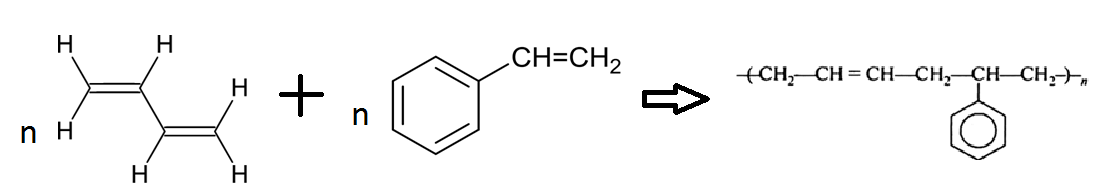
Buna-S is formed from the polymerization of butadiene and styrene in $1:3$. In this process peroxide is used as a catalyst at $5^\circ C$ therefore the formed product is also known as cold rubber. The vulcanization of this rubber is done with sulphur for better elasticity.
It has good resistance and long life stability when protected from additives.
They are used in manufacture of conveyor belts, shoe soles and electrical insulation.
Hence option (C) is correct, The monomers of Buna-S rubber are Styrene and butadiene.
Note:
Buna-S is called from different names such as cold rubber as it is manufactured under $5^\circ C$, styrene butadiene rubber (SBR) and 1-3,butadiene is IUPAC name of Buna-S.
Complete step by step answer:
Buna-S is a synthetic polymer made up from the combination of butadiene and styrene. It is a copolymer of $75\% $ butadiene and $25\% $ styrene.
Butadiene

Styrene

The polymerization reaction involved in the formation of Buna-S:

Buna-S is formed from the polymerization of butadiene and styrene in $1:3$. In this process peroxide is used as a catalyst at $5^\circ C$ therefore the formed product is also known as cold rubber. The vulcanization of this rubber is done with sulphur for better elasticity.
It has good resistance and long life stability when protected from additives.
They are used in manufacture of conveyor belts, shoe soles and electrical insulation.
Hence option (C) is correct, The monomers of Buna-S rubber are Styrene and butadiene.
Note:
Buna-S is called from different names such as cold rubber as it is manufactured under $5^\circ C$, styrene butadiene rubber (SBR) and 1-3,butadiene is IUPAC name of Buna-S.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




