
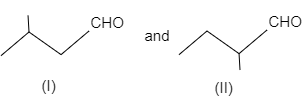
The most likely acid-catalyzed aldol condensation products of each of the two aldehydes (I) and (II) will respectively be

Answer
573.6k+ views
Hint: Aldol condensation reaction is a condensation reaction involving aldehydes and ketones. It can be acid catalyzed or base catalyzed. Not all aldehydes and ketones can undergo aldol condensation.
Complete Solution :
- Aldol condensation reaction is a very important name reaction. It is the reaction between an enolizable aldehyde and enolizable ketone occurring in the presence of an acid or base catalyst in aqueous medium at high temperature, and further the reaction tends to give a beta unsaturated aldehyde (i.e. unsaturation occurs on beta carbon, which is the second carbon atom attached to a functional group) or a beta unsaturated ketone, respectively as the product.
- Acid catalyzed aldol condensation reaction involves $[1,2]$ addition reaction between an enol and an aldehyde or ketone. In acid catalyzed aldol condensation, the catalyst used is usually a hydrogen ion (from water). Aldol condensation reactions can occur between or two aldehydes or ketones provided they have alpha hydrogen in them. It can also occur between an aldehyde and a ketone where one should have alpha hydrogen. This type of reaction is called cross aldol condensation.
Coming to the question,
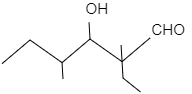
- In figure (I), the product of the reaction will undergo dehydration as the carbon-carbon double bond is in conjugation with $C=O$ double bond and this provides the product stability. The reaction is shown as follows:
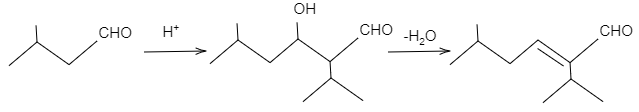
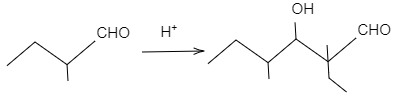
In figure (II), the product of the reaction will not undergo dehydration as the carbon-carbon double bond is not in conjugation with $C=O$ double bond due to presence of quaternary carbon atoms. The reaction is shown as follows:
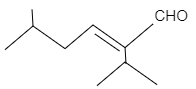
Hence, the products of figure (I) and (II) are.
Note: You should always remember that, for an aldol condensation reaction to occur there must be at least one alpha hydrogen in the aldehyde or ketone. It is to be noted that, in base catalyzed aldol condensation, hydroxyl ion acts as the nucleophile while in acid catalyzed reaction the hydrogen ion acts as the nucleophile.
Complete Solution :
- Aldol condensation reaction is a very important name reaction. It is the reaction between an enolizable aldehyde and enolizable ketone occurring in the presence of an acid or base catalyst in aqueous medium at high temperature, and further the reaction tends to give a beta unsaturated aldehyde (i.e. unsaturation occurs on beta carbon, which is the second carbon atom attached to a functional group) or a beta unsaturated ketone, respectively as the product.
- Acid catalyzed aldol condensation reaction involves $[1,2]$ addition reaction between an enol and an aldehyde or ketone. In acid catalyzed aldol condensation, the catalyst used is usually a hydrogen ion (from water). Aldol condensation reactions can occur between or two aldehydes or ketones provided they have alpha hydrogen in them. It can also occur between an aldehyde and a ketone where one should have alpha hydrogen. This type of reaction is called cross aldol condensation.
Coming to the question,
- In figure (I), the product of the reaction will undergo dehydration as the carbon-carbon double bond is in conjugation with $C=O$ double bond and this provides the product stability. The reaction is shown as follows:

In figure (II), the product of the reaction will not undergo dehydration as the carbon-carbon double bond is not in conjugation with $C=O$ double bond due to presence of quaternary carbon atoms. The reaction is shown as follows:

Hence, the products of figure (I) and (II) are.


Note: You should always remember that, for an aldol condensation reaction to occur there must be at least one alpha hydrogen in the aldehyde or ketone. It is to be noted that, in base catalyzed aldol condensation, hydroxyl ion acts as the nucleophile while in acid catalyzed reaction the hydrogen ion acts as the nucleophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




