
The name of the alkane isomer of cis-3-hexene is:
A. 2-methylpentane
B. 3-methylpentane
C. n-hexane
D. cyclohexane
Answer
568.8k+ views
Hint: To answer this question we need to know the molecular formula of all the compounds. Knowing the structure can make the task simpler and you can get to the answer easily and quickly. With the formula we can count the number of carbon and hydrogen atoms and comparing it with cis-3-hexene we can get the answer.
Step by step answer: To answer this question, we need to understand isomerism and alkane isomer. Isomerism is the phenomenon in which more than one compound has the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulas but have different properties and the arrangement of atoms in the molecule are called isomers. There are two types of isomerism which are Structural isomerism and Stereoisomerism. Structural isomerism can be further divided into chain, position and functional isomers and Stereoisomers can be divided into geometric and optical isomers. Structural isomerism refers to the compounds that differ in their connectivity between the atoms and stereo isomerism refers to the compounds with same exact molecular formula and connectivity but differ in the arrangement of atoms in three dimensional space.
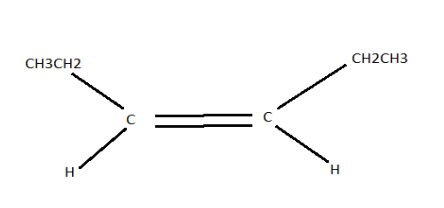
Alkane means compounds consisting of hydrogen and carbon atoms arranged in a structure in which all the carbon-carbon bonds are single. Therefore, alkane isomers are hydrogen and carbon compounds that have isomeric properties. Now, to answer this question we need to know the structure and molecular formula of cis-3-hexene. Its molecular formula is \[{C_6}{H_{14}}\] . Given below is the structure of cis-3-hexane.
Now, let’s see the structure and molecular formula of compounds given in options.
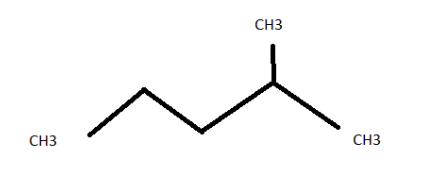
2-methylpentane ( \[{C_6}{H_{14}}\] )
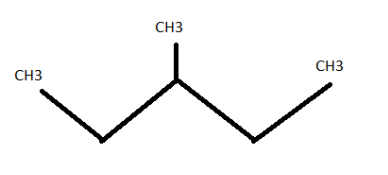
3-methylpentane ( \[{C_6}{H_{14}}\] )
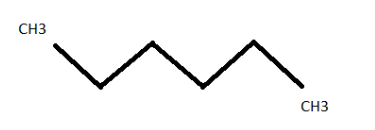
n-hexane ( \[{C_6}{H_{14}}\] )
Cyclohexane ( \[{C_6}{H_{12}}\] )
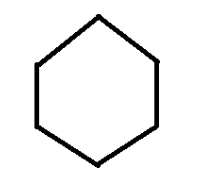
From the structure of the compounds and the molecular formula we get to know that Cyclohexane has the same molecular formula as cis-3-hexene. Therefore, alkane isomer of cis-3-hexene is cyclohexane. Here, we can see that both cyclohexane and cis-3-hexene have same molecular formula but different structures. Therefore, satisfying the property of isomerism especially structural isomerism.
Therefore, the answer is option D.
Note: The structure and molecular formula are an important part of organic chemistry. Every prefix used in naming is different and helps determine its structure. Understanding the meaning of the prefixes is also important.
Step by step answer: To answer this question, we need to understand isomerism and alkane isomer. Isomerism is the phenomenon in which more than one compound has the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulas but have different properties and the arrangement of atoms in the molecule are called isomers. There are two types of isomerism which are Structural isomerism and Stereoisomerism. Structural isomerism can be further divided into chain, position and functional isomers and Stereoisomers can be divided into geometric and optical isomers. Structural isomerism refers to the compounds that differ in their connectivity between the atoms and stereo isomerism refers to the compounds with same exact molecular formula and connectivity but differ in the arrangement of atoms in three dimensional space.
Alkane means compounds consisting of hydrogen and carbon atoms arranged in a structure in which all the carbon-carbon bonds are single. Therefore, alkane isomers are hydrogen and carbon compounds that have isomeric properties. Now, to answer this question we need to know the structure and molecular formula of cis-3-hexene. Its molecular formula is \[{C_6}{H_{14}}\] . Given below is the structure of cis-3-hexane.

Now, let’s see the structure and molecular formula of compounds given in options.
2-methylpentane ( \[{C_6}{H_{14}}\] )

3-methylpentane ( \[{C_6}{H_{14}}\] )

n-hexane ( \[{C_6}{H_{14}}\] )

Cyclohexane ( \[{C_6}{H_{12}}\] )

From the structure of the compounds and the molecular formula we get to know that Cyclohexane has the same molecular formula as cis-3-hexene. Therefore, alkane isomer of cis-3-hexene is cyclohexane. Here, we can see that both cyclohexane and cis-3-hexene have same molecular formula but different structures. Therefore, satisfying the property of isomerism especially structural isomerism.
Therefore, the answer is option D.
Note: The structure and molecular formula are an important part of organic chemistry. Every prefix used in naming is different and helps determine its structure. Understanding the meaning of the prefixes is also important.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




