
The number of chain isomers possible for hydrocarbon ${C_5}{H_{12}}$ is:
A.3
B.5
C.4
D.6
Answer
585k+ views
Hint: Isomers are the compounds which have the same molecular formula but the arrangement of the atoms in the space is different. The physical and chemical properties of the isomers are not the same; they vary. The phenomenon of the existence of a compound in more than one form of isomers is known as isomerism.
Complete step by step answer:
The isomers are of different type’s functional, position, chain isomers. Chain isomers are the isomers which have the same molecular formula and are made up of carbon atoms but the atomic arrangement of these carbon atoms in space is different.
The 5 carbon atoms straight chain alkane is known as pentane. The molecular formula of pentane is ${C_5}{H_{12}}$ and its molar mass is 72 g/ mole. It is volatile in nature. The structure of pentane is given below:
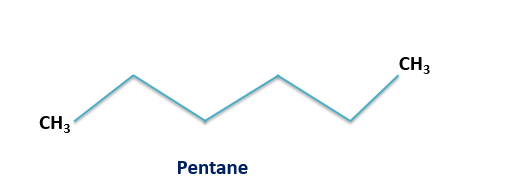
Thus the total number of chain isomers possible for the hydrocarbon ${C_5}{H_{12}}$ is 3 and hence option A is the correct answer. These are n- pentane, isopentane and neopentane. The IUPAC names for these isomers are pentane, 2-methylbutane and 2, 2-dimethylpropane respectively. The structures for these isomers are given below:
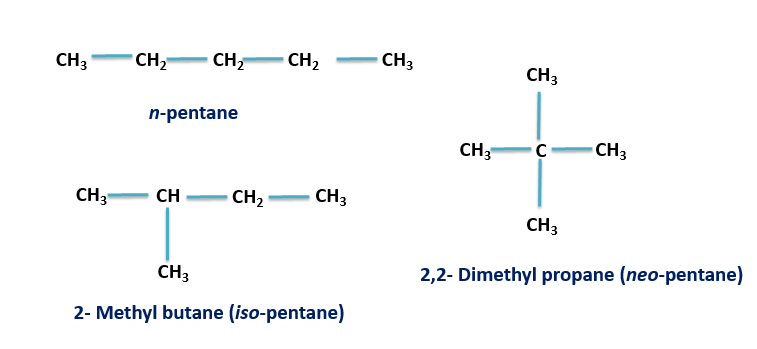
Pentane is used as a solvent and a refrigerant. It is also used in the creation of foam of polystyrene which in turn is used to make insulation materials. If the pentane is inhaled by mistake it leads to the irritation of the nose and throat.
So therefore option A is the correct answer.
Note:
Pentane is heavier than air and is extremely flammable in nature. The flash fires can be caused due to this property of pentane since it is heavier than air the vapours of it gets collected in the containers and results in the creation of the persistent atmosphere which is flammable in nature.
Complete step by step answer:
The isomers are of different type’s functional, position, chain isomers. Chain isomers are the isomers which have the same molecular formula and are made up of carbon atoms but the atomic arrangement of these carbon atoms in space is different.
The 5 carbon atoms straight chain alkane is known as pentane. The molecular formula of pentane is ${C_5}{H_{12}}$ and its molar mass is 72 g/ mole. It is volatile in nature. The structure of pentane is given below:

Thus the total number of chain isomers possible for the hydrocarbon ${C_5}{H_{12}}$ is 3 and hence option A is the correct answer. These are n- pentane, isopentane and neopentane. The IUPAC names for these isomers are pentane, 2-methylbutane and 2, 2-dimethylpropane respectively. The structures for these isomers are given below:

Pentane is used as a solvent and a refrigerant. It is also used in the creation of foam of polystyrene which in turn is used to make insulation materials. If the pentane is inhaled by mistake it leads to the irritation of the nose and throat.
So therefore option A is the correct answer.
Note:
Pentane is heavier than air and is extremely flammable in nature. The flash fires can be caused due to this property of pentane since it is heavier than air the vapours of it gets collected in the containers and results in the creation of the persistent atmosphere which is flammable in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




