
The number of electrons, protons and neutrons in an atom whose atomic number is 11 and mass number 23 will be:
(A) 11, 11, 12
(B)11, 12, 11
(C) 12, 11, 11
(D) 23, 11, 23
Answer
583.8k+ views
Hint: Sodium (Na) is the element in the periodic table whose atomic number is 11. To solve this question it is important to remember the representation of the element in the periodic table and definition as well as the difference between atomic number and mass number.
Complete step by step solution:
First, let’s see the representation atom structure in the periodic table.
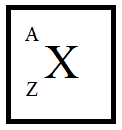
Where X is the element
A is the mass number
Z is the atomic number
The mass number can be defined as the mass of the atom. The number of protons present in the nucleus is known as the atomic number.
The relationship between A and Z is,
A= Z+ N………………………….equation 1
Where N is the number of neutrons.
Atomic number = Number of protons = Number of electrons
Z= P= e
Where P is the number of protons
e is the number of electrons.
We know sodium is the element whose atomic mass is 11.
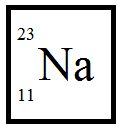
A= 23
Z= 11
-The number of neutrons in sodium:
Substitute value of A and Z in equation 1
A= Z+ N
23 =11+N
N = 23-11
N= 12
Therefore, the number of neutrons is equal to 12.
-The number of protons in sodium
From equation 2, we know that Z= P.
Therefore P is 11.
-The number of electrons
From equation 2, we know that P = e
Therefore e is 11
Thus the correct answer is option (A).
Note: Two main points that we have to keep in mind in order to not go wrong with this kind of questions:
-Remember the notation given to the atomic number and mass number as well as both equation 1 and 2.
-Always carefully answer in the order in which they have asked for the value. For example in our question, the number of electrons, protons and neutrons are asked and the answer is 11, 11, 12 but if we write 11,12,11 then, the answer will be wrong.
Complete step by step solution:
First, let’s see the representation atom structure in the periodic table.
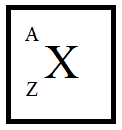
Where X is the element
A is the mass number
Z is the atomic number
The mass number can be defined as the mass of the atom. The number of protons present in the nucleus is known as the atomic number.
The relationship between A and Z is,
A= Z+ N………………………….equation 1
Where N is the number of neutrons.
Atomic number = Number of protons = Number of electrons
Z= P= e
Where P is the number of protons
e is the number of electrons.
We know sodium is the element whose atomic mass is 11.
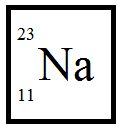
A= 23
Z= 11
-The number of neutrons in sodium:
Substitute value of A and Z in equation 1
A= Z+ N
23 =11+N
N = 23-11
N= 12
Therefore, the number of neutrons is equal to 12.
-The number of protons in sodium
From equation 2, we know that Z= P.
Therefore P is 11.
-The number of electrons
From equation 2, we know that P = e
Therefore e is 11
Thus the correct answer is option (A).
Note: Two main points that we have to keep in mind in order to not go wrong with this kind of questions:
-Remember the notation given to the atomic number and mass number as well as both equation 1 and 2.
-Always carefully answer in the order in which they have asked for the value. For example in our question, the number of electrons, protons and neutrons are asked and the answer is 11, 11, 12 but if we write 11,12,11 then, the answer will be wrong.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




