
The number of isomers possible for propane is $2.$
A. True
B. False
Answer
582.6k+ views
Hint: In Chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecules has the same number of atoms of each element, but with logically distinct bonds between them. Isomers arise when a particular compound can be represented in a long or branched structural chain with the same number of carbon and other atoms.
Complete step by step answer: In alkanes including methane, ethane and propane there is only one possible arrangement of the bonding atoms. They do not have sufficient numbers of carbon atoms to form a branched chain of isomers. In propane, the third carbon has to be attached to either of the other two carbons forming a three carbon chain with eight additional bonding sites, each bonded to a hydrogen atom. Propane is a saturated molecule.${{\text{C}}_3}{{\text{H}}_8}$ is completely unambiguous.
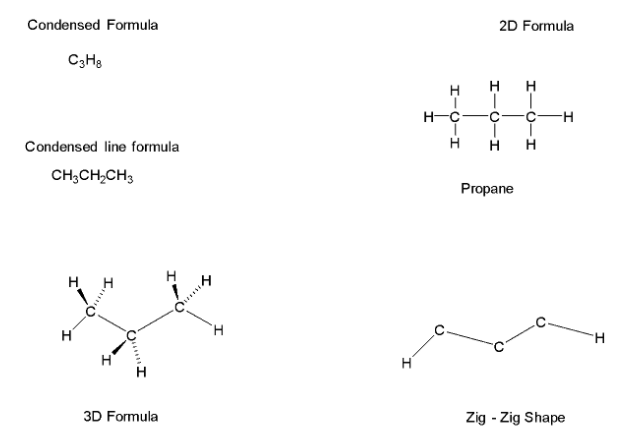
It does not even have $2$ isomers, chain isomerism in alkanes starts from Butane which contains four
carbon atoms.
So, the correct answer is “Option B”.
Additional Information: Propane is an alkane ${{\text{C}}_3}{{\text{H}}_{8,}}({\text{C}}{{\text{H}}_3}{\text{ - C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3})$ containing two methyl groups $\left( {{\text{C}}{{\text{H}}_3} - } \right)$ bonded to a central ${\text{C}}{{\text{H}}_2} - $, so it present some symmetry related to central carbon. This means that the propane does not have isomers. Isomers can be formed from alkanes having at least four carbon atoms. In case of butane $\left( {{{\text{C}}_4}{{\text{H}}_{10}}} \right)$. So you may have the linear Butane and Isobutane.
Note: While finding the isomers of a given compound firstly draw the structure chain of the compound using the structural formula of the compound. Then try to modify the place of carbon atoms without making any change in their number and draw the different structures as possible. Functional isomers are structural isomers which have different functional groups resulting in significantly different chemical and physical properties.
Complete step by step answer: In alkanes including methane, ethane and propane there is only one possible arrangement of the bonding atoms. They do not have sufficient numbers of carbon atoms to form a branched chain of isomers. In propane, the third carbon has to be attached to either of the other two carbons forming a three carbon chain with eight additional bonding sites, each bonded to a hydrogen atom. Propane is a saturated molecule.${{\text{C}}_3}{{\text{H}}_8}$ is completely unambiguous.

It does not even have $2$ isomers, chain isomerism in alkanes starts from Butane which contains four
carbon atoms.
So, the correct answer is “Option B”.
Additional Information: Propane is an alkane ${{\text{C}}_3}{{\text{H}}_{8,}}({\text{C}}{{\text{H}}_3}{\text{ - C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3})$ containing two methyl groups $\left( {{\text{C}}{{\text{H}}_3} - } \right)$ bonded to a central ${\text{C}}{{\text{H}}_2} - $, so it present some symmetry related to central carbon. This means that the propane does not have isomers. Isomers can be formed from alkanes having at least four carbon atoms. In case of butane $\left( {{{\text{C}}_4}{{\text{H}}_{10}}} \right)$. So you may have the linear Butane and Isobutane.
Note: While finding the isomers of a given compound firstly draw the structure chain of the compound using the structural formula of the compound. Then try to modify the place of carbon atoms without making any change in their number and draw the different structures as possible. Functional isomers are structural isomers which have different functional groups resulting in significantly different chemical and physical properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




