
The number of P-O-P bond in cyclic metaphosphoric acid is:
A. zero
B. two
C. three
D. four
Answer
558.9k+ views
Hint: phosphoric acid contains three atoms of hydrogen, one atom of phosphorus and four atoms of oxygen.Meta-phosphoric acid is corrosive in nature, inorganic, cyclic polyphosphate which is formed from bonded phosphoric acid units. It can also be deprotonated to cyclic metaphosphate anions formation and it has many applications in biochemistry, agriculture, pharmacy, and chemical research.
Complete step by step answer:
We need to find the number of P-O-P bonds in the cyclic metaphosphoric acid.
Meta-Phosphoric acid:
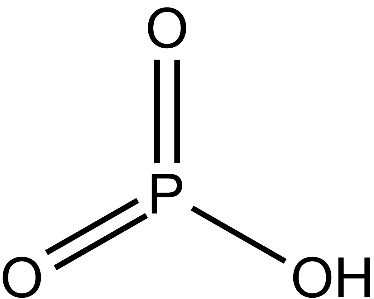
- The above figure shows the structure of meta phosphoric acid.
- Meta-phosphoric acid is the oxo-acid of phosphorus. It forms a double bond with the O atom and also forms a single bond with the OH group.
- The number of P-O-P bonds in cyclic metaphosphoric acid is 3 as shown in the figure.
Tri-meta-phosphoric acid:
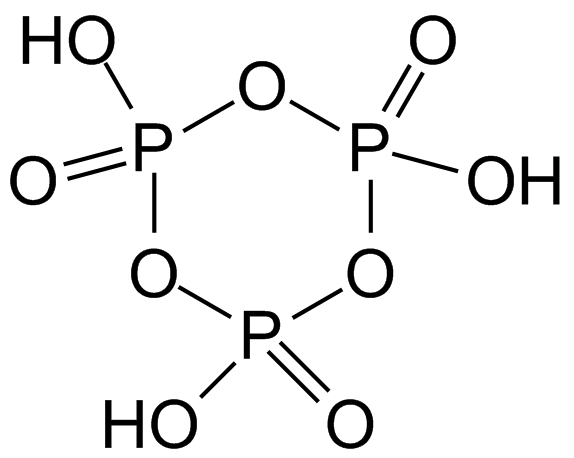
The figure above shows the structure of cyclic meta phosphoric acid.
- Trimetaphosphoric acid is also an oxo-acid of phosphorus.
- Cyclometa-phosphoric acid:
- It is a tribasic compound.
The correct answer is option “C” .
Note: Whenever there is a question asked to tell the number of bonds of any acid or compound then always the structure should be made and then the bonds can be easily seen, as for this question, there are 3 P-O-P bonds. Cyclo tri-phosphoric acid is actually cyclic anhydride of tri-phosphoric acid. It is a phosphorus oxo-acid, which is an inorganic heterocyclic compound and a cyclic phosphorus acid anhydride.
- Meta-phosphoric acid in around 2 percent concentration, as suggested by these people Fujita and Iwatake, helps to protect vitamin C in solution against atmospheric oxidation, even in the existence of added copper, and also takes protective action against oxidation in the existence of trichloroacetic acid.
Complete step by step answer:
We need to find the number of P-O-P bonds in the cyclic metaphosphoric acid.
Meta-Phosphoric acid:

- The above figure shows the structure of meta phosphoric acid.
- Meta-phosphoric acid is the oxo-acid of phosphorus. It forms a double bond with the O atom and also forms a single bond with the OH group.
- The number of P-O-P bonds in cyclic metaphosphoric acid is 3 as shown in the figure.
Tri-meta-phosphoric acid:

The figure above shows the structure of cyclic meta phosphoric acid.
- Trimetaphosphoric acid is also an oxo-acid of phosphorus.
- Cyclometa-phosphoric acid:
- It is a tribasic compound.
The correct answer is option “C” .
Note: Whenever there is a question asked to tell the number of bonds of any acid or compound then always the structure should be made and then the bonds can be easily seen, as for this question, there are 3 P-O-P bonds. Cyclo tri-phosphoric acid is actually cyclic anhydride of tri-phosphoric acid. It is a phosphorus oxo-acid, which is an inorganic heterocyclic compound and a cyclic phosphorus acid anhydride.
- Meta-phosphoric acid in around 2 percent concentration, as suggested by these people Fujita and Iwatake, helps to protect vitamin C in solution against atmospheric oxidation, even in the existence of added copper, and also takes protective action against oxidation in the existence of trichloroacetic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




