
The number of possible isomers of butene are:
(A) $ 3 $
(B) $ 2 $
(C) $ 4 $
(D) $ 5 $
Answer
510k+ views
Hint: The concept of isomerism occurs when two or more molecules have the same chemical formula but different chemical structures. Isomers are chemical compounds with similar chemical formulae that vary in properties and atom structure in the molecule. As a result, compounds that show isomerism are referred to as isomers.
Complete answer:
Butene is an alkene with the formula $ {C_4}{H_8} $ that is also known as butylene. Butene may refer to all of the compounds individually. They are colourless gases that are present in crude oil as a minor constituent in amounts that make extraction impossible. Butene is made by catalytic cracking of long-chain hydrocarbons left over from crude oil refining. Fractional distillation is used to remove butene from a mixture of products produced by cracking.
Butene can be used as a monomer for polybutene, but it is more costly than polypropylene and other polymers with shorter carbon chains.
There are four geometrical isomers of Butene:
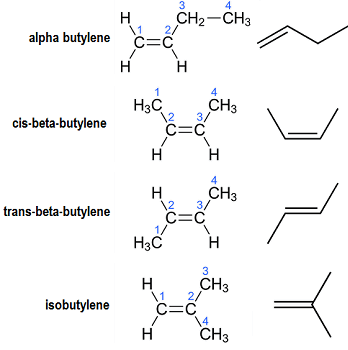
Some organic compounds, such as cyclobutane and methylcyclopropane, have the formula $ {C_4}{H_8} $ , but they are not alkenes and do not come under the name butene. There are also four-carbon cyclic alkenes, such as cyclobutene and two isomers of methylcyclopropene, but they don't have the formula $ {C_4}{H_8} $ and aren't covered here.
So option (c) is correct.
Note:
1-Butene is a stable compound that readily polymerizes to polybutene. Its primary use is as a commoner in the manufacture of specific types of polyethylene, such as linear low-density polyethylene (LLDPE). Polypropylene resins, butylene oxide, and butanone have all used it as a precursor.
Complete answer:
Butene is an alkene with the formula $ {C_4}{H_8} $ that is also known as butylene. Butene may refer to all of the compounds individually. They are colourless gases that are present in crude oil as a minor constituent in amounts that make extraction impossible. Butene is made by catalytic cracking of long-chain hydrocarbons left over from crude oil refining. Fractional distillation is used to remove butene from a mixture of products produced by cracking.
Butene can be used as a monomer for polybutene, but it is more costly than polypropylene and other polymers with shorter carbon chains.
There are four geometrical isomers of Butene:

Some organic compounds, such as cyclobutane and methylcyclopropane, have the formula $ {C_4}{H_8} $ , but they are not alkenes and do not come under the name butene. There are also four-carbon cyclic alkenes, such as cyclobutene and two isomers of methylcyclopropene, but they don't have the formula $ {C_4}{H_8} $ and aren't covered here.
So option (c) is correct.
Note:
1-Butene is a stable compound that readily polymerizes to polybutene. Its primary use is as a commoner in the manufacture of specific types of polyethylene, such as linear low-density polyethylene (LLDPE). Polypropylene resins, butylene oxide, and butanone have all used it as a precursor.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




