
The number of resonating structures of benzene are _________.
(A) 1
(B) 2
(C) 3
(D) 4
Answer
579k+ views
Hint: Resonance structures are hypothetical and do not represent any real molecule. The real structure of a molecule is a hybrid of the resonating structure.
Complete step by step answer:
Let us discuss the structure of benzene.
The structure of benzene cannot be explained by a single Lewis structure.
The structure of benzene is cyclic. It contains C-C single and C=C double bond and represented as
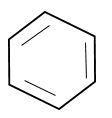
As per above representation benzene exhibits two bond lengths due to C-C and C=C bonds.
But it was experimentally determined that benzene has uniform bond length i.e., $139pm.$
This value is intermediate between C-C single $[154pm]$ and C=C double $[134pm]$bonds.
Therefore, benzene can be represented by energetically identical structure I and II.
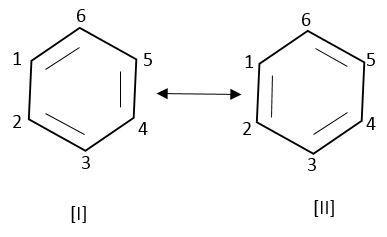
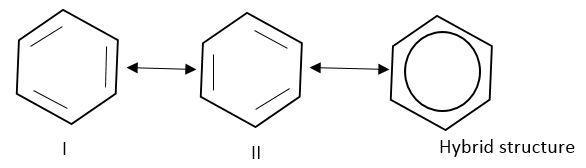
Therefore, the structure of benzene cannot be represented by any of their structure.
It is a hybrid of these two resonating structures.
Therefore, from the above explanation the correct option is (B) 2.
Additional Information:
The energy of resonance hybrid is lower than that of its resonating structure.
The difference in energy between hybrid structure and resonating structure is called resonance energy.
The more the number of resonating structures the more is resonance energy.
Note:
In benzene each C-atom is $s{p^2}$ hybridized.
Two hybridized $s{p^2}$ orbitals form $\sigma $ bond and unhybridized orbitals of C-atom form $\pi $ bond.
With adjacent C-atoms by lateral overlap.
This explains equal possibility for formation of ${C_1} - {C_2},{C_3} - {C_4},{C_5} - {C_6}$$\pi $ bond or ${C_2} - {C_3},{C_4} - {C_5},{C_6} - {C_1}$$\pi $ bond.
The hybrid structure is represented by a circle inside the ring.
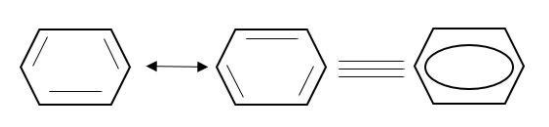
Complete step by step answer:
Let us discuss the structure of benzene.
The structure of benzene cannot be explained by a single Lewis structure.
The structure of benzene is cyclic. It contains C-C single and C=C double bond and represented as

As per above representation benzene exhibits two bond lengths due to C-C and C=C bonds.
But it was experimentally determined that benzene has uniform bond length i.e., $139pm.$
This value is intermediate between C-C single $[154pm]$ and C=C double $[134pm]$bonds.
Therefore, benzene can be represented by energetically identical structure I and II.

Therefore, the structure of benzene cannot be represented by any of their structure.
It is a hybrid of these two resonating structures.

Therefore, from the above explanation the correct option is (B) 2.
Additional Information:
The energy of resonance hybrid is lower than that of its resonating structure.
The difference in energy between hybrid structure and resonating structure is called resonance energy.
The more the number of resonating structures the more is resonance energy.
Note:
In benzene each C-atom is $s{p^2}$ hybridized.
Two hybridized $s{p^2}$ orbitals form $\sigma $ bond and unhybridized orbitals of C-atom form $\pi $ bond.
With adjacent C-atoms by lateral overlap.
This explains equal possibility for formation of ${C_1} - {C_2},{C_3} - {C_4},{C_5} - {C_6}$$\pi $ bond or ${C_2} - {C_3},{C_4} - {C_5},{C_6} - {C_1}$$\pi $ bond.
The hybrid structure is represented by a circle inside the ring.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




