
The number of \[S - O - S\] bonds in cyclic \[S{O_3}\] is:
A. \[1\]
B. \[2\]
C. \[3\]
D. none of the above
Answer
543.6k+ views
Hint:Here, we need to find the number of bonds so for that first we need to draw the figure of \[S{O_3}\] and with the help of that figure we can easily observe and count the total number of bonds.
Complete step-by-step answer:Here, we need to find the total number of \[S - O - S\] bonds in cyclic Sulfur trioxide.
Sulfur trioxide:
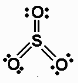
The above figure shows the structure of sulfur trioxide.
Sulfur trioxide can be defined as a chemical compound which is represented by chemical formula \[S{O_3}\]. Sulfur trioxide is present in a number of modifications that vary in molecular species and crystalline form. In the liquid form, it is colourless and fumes in air at ambient conditions. It is a very highly reactive substance and also a strong oxidising agent and it also acts as a fire hazard. It is a thermodynamically not stable compound with respect to selenium dioxide.
Cyclic Sulfur trioxide:
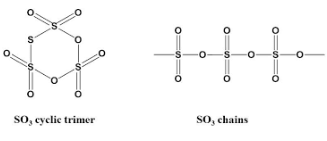
The above figure shows the structure of cyclic Sulfur trioxide and the chains used in creation of the compound.
The cyclic trimer of \[S{O_3}\] has \[3\] \[S - O - S\] bonds as we can see in the figure.
Additional Information:
Sulfur trioxide (also spelled as sulphur trioxide) is the chemical compound having the formula \[S{O_3}\], with a relatively narrow liquid range. In its gaseous form, this species is a highly pollutant, and being the primary agent in acid rain.
It is formed on an industrial scale as a precursor to sulphuric acid, and is also called as sulphuric anhydride.
Hence, Option C is correct.
Note:Whenever there is a question asked to tell the number of bonds of any acid or compound then always the structure should be made and then the bonds can be easily visible, as for this question, there are \[3\] \[S - O - S\] bonds.
Complete step-by-step answer:Here, we need to find the total number of \[S - O - S\] bonds in cyclic Sulfur trioxide.
Sulfur trioxide:

The above figure shows the structure of sulfur trioxide.
Sulfur trioxide can be defined as a chemical compound which is represented by chemical formula \[S{O_3}\]. Sulfur trioxide is present in a number of modifications that vary in molecular species and crystalline form. In the liquid form, it is colourless and fumes in air at ambient conditions. It is a very highly reactive substance and also a strong oxidising agent and it also acts as a fire hazard. It is a thermodynamically not stable compound with respect to selenium dioxide.
Cyclic Sulfur trioxide:

The above figure shows the structure of cyclic Sulfur trioxide and the chains used in creation of the compound.
The cyclic trimer of \[S{O_3}\] has \[3\] \[S - O - S\] bonds as we can see in the figure.
Additional Information:
Sulfur trioxide (also spelled as sulphur trioxide) is the chemical compound having the formula \[S{O_3}\], with a relatively narrow liquid range. In its gaseous form, this species is a highly pollutant, and being the primary agent in acid rain.
It is formed on an industrial scale as a precursor to sulphuric acid, and is also called as sulphuric anhydride.
Hence, Option C is correct.
Note:Whenever there is a question asked to tell the number of bonds of any acid or compound then always the structure should be made and then the bonds can be easily visible, as for this question, there are \[3\] \[S - O - S\] bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




