
The number of $S - S\,$ bonds in sulphur trioxide trimer, $\left( {{S_3}{O_9}} \right)$ is:
A. three
B. two
C. one
D. zero
Answer
594.3k+ views
Hint: Sulphur trioxide trimer has the chemical formula $\left( {{{\rm{S}}_{\rm{3}}}{{\rm{O}}_{\rm{9}}}} \right)$, that is it has three sulphur atoms and nine oxygen atoms. The chemical formula of Sulphur trioxide is ${\rm{S}}{{\rm{O}}_{\rm{3}}}$.
Complete step by step answer:
We can draw the structure of Sulphur trioxide trimer as follows.
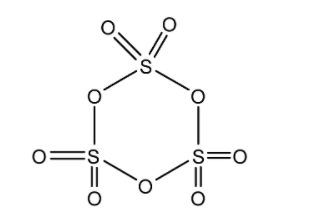
In the structure of the Sulphur trioxide trimer above, we can see that all the three sulphur atoms are bonded with oxygen atoms on all the sides. We see that there is no bond taking place between the sulphur atoms. So, we can conclude that the number of $S - S\,$ bonds in sulphur trioxide trimer, $\left( {{S_3}{O_9}} \right)$ is zero.
So, out of the given four options, option D is the correct option.
Additional Information:
Sulfur trioxide is a colourless liquid. It can also exist as fibre-like crystals or like ice or also as a gas. When Sulphur trioxide is exposed to air, it takes up water molecules and releases white fumes. Sulfur trioxide reacts with water forming sulphuric acid. Sulfur trioxide is also called sulfuric oxide and also as sulfuric anhydride. It is used for production of sulphuric acid and other chemicals as well as explosives. Sulphuric acid is a colourless, clear oily liquid and it is very corrosive. It is also called as a sulphite acid hydrogen sulfate and also sometimes as battery acids. It is used in the production of explosives, fertilizers, and other acids in petroleum purification. It is also used in the pickling of metal and in the lead-acid batteries.
Note:
There exist no bonds between the sulphur atoms in sulfur trioxide trimer. Three S-O-S bonds and six S-O bonds exist in the sulfur trioxide trimer compound.
Complete step by step answer:
We can draw the structure of Sulphur trioxide trimer as follows.

In the structure of the Sulphur trioxide trimer above, we can see that all the three sulphur atoms are bonded with oxygen atoms on all the sides. We see that there is no bond taking place between the sulphur atoms. So, we can conclude that the number of $S - S\,$ bonds in sulphur trioxide trimer, $\left( {{S_3}{O_9}} \right)$ is zero.
So, out of the given four options, option D is the correct option.
Additional Information:
Sulfur trioxide is a colourless liquid. It can also exist as fibre-like crystals or like ice or also as a gas. When Sulphur trioxide is exposed to air, it takes up water molecules and releases white fumes. Sulfur trioxide reacts with water forming sulphuric acid. Sulfur trioxide is also called sulfuric oxide and also as sulfuric anhydride. It is used for production of sulphuric acid and other chemicals as well as explosives. Sulphuric acid is a colourless, clear oily liquid and it is very corrosive. It is also called as a sulphite acid hydrogen sulfate and also sometimes as battery acids. It is used in the production of explosives, fertilizers, and other acids in petroleum purification. It is also used in the pickling of metal and in the lead-acid batteries.
Note:
There exist no bonds between the sulphur atoms in sulfur trioxide trimer. Three S-O-S bonds and six S-O bonds exist in the sulfur trioxide trimer compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




