
The number of \[S - S\] bonds in sulphur trioxide trimer \[\left( {{S_3}{O_9}} \right)\] is:
A.3
B.2
C.1
D.0
Answer
569.4k+ views
Hint:
In chemistry, a trimer is a molecule or an anion formed by the combination or association of three identical or simpler molecules or ions of the same substance .They are linked by hydrogen bonds or covalent bonds. Raffinose is a trimer composed of the monomers glucose, fructose and galactose.
Complete step by step answer:
The structure of sulphur trioxide trimer \[\left( {{S_3}{O_9}} \right)\] is
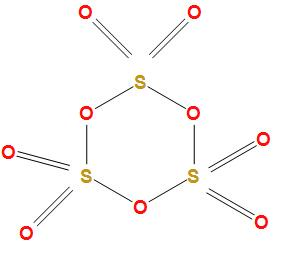
It is a cyclic trimer. In the sulphur trioxide trimer \[\left( {{S_3}{O_9}} \right)\] we can see that there is no \[S - S\] bond. The sulphur atoms are double and single bonded with oxygen atoms alternatively.
Therefore, the total number of \[S - S\] bonds in sulphur trioxide trimer is 0.
Thus, the correct option is (D).
Note:There are some allotropes of sulphur.
-Rhombic sulphur: - This form is stable at ordinary temperature. All other forms change into the variety of standing. It is obtained by dissolving roll sulphur in carbon disulphide and then evaporating the solution. After evaporating the solution, octahedral crystals of rhombic sulphur are obtained. Its melting point is \[{112.8^ \circ }C\] and its density is 2.07 gm/ml. It is soluble in carbon disulphide but insoluble in water.
-Monoclinic sulphur: - Some ordinary sulphur is taken in a china dish and heated to melt. On cooling a crust is formed on the surface. A few holes are made on the crust and liquid and below it is poured off. Needle shaped crystals of monoclinic sulphur are formed which are seen on removing the crust. If we heat rhombic sulphur at \[{95.5^ \circ }C\] it gets converted to monoclinic sulphur.
-Plastic sulphur: - It is formed by boiling ordinary roll sulphur in a test tube and pouring the boiling mass into cold water. As a result, rubber like plastic sulphur is formed.
-Colloidal sulphur: - It is obtained by passing hydrogen sulphide through a solution of some oxidizing agents like nitric acid, potassium permanganate, sulphur dioxide solution etc.
\[2HN{O_3} + {H_2}S \to 2N{O_2} + 2{H_2}O + S \downarrow \]
In chemistry, a trimer is a molecule or an anion formed by the combination or association of three identical or simpler molecules or ions of the same substance .They are linked by hydrogen bonds or covalent bonds. Raffinose is a trimer composed of the monomers glucose, fructose and galactose.
Complete step by step answer:
The structure of sulphur trioxide trimer \[\left( {{S_3}{O_9}} \right)\] is

It is a cyclic trimer. In the sulphur trioxide trimer \[\left( {{S_3}{O_9}} \right)\] we can see that there is no \[S - S\] bond. The sulphur atoms are double and single bonded with oxygen atoms alternatively.
Therefore, the total number of \[S - S\] bonds in sulphur trioxide trimer is 0.
Thus, the correct option is (D).
Note:There are some allotropes of sulphur.
-Rhombic sulphur: - This form is stable at ordinary temperature. All other forms change into the variety of standing. It is obtained by dissolving roll sulphur in carbon disulphide and then evaporating the solution. After evaporating the solution, octahedral crystals of rhombic sulphur are obtained. Its melting point is \[{112.8^ \circ }C\] and its density is 2.07 gm/ml. It is soluble in carbon disulphide but insoluble in water.
-Monoclinic sulphur: - Some ordinary sulphur is taken in a china dish and heated to melt. On cooling a crust is formed on the surface. A few holes are made on the crust and liquid and below it is poured off. Needle shaped crystals of monoclinic sulphur are formed which are seen on removing the crust. If we heat rhombic sulphur at \[{95.5^ \circ }C\] it gets converted to monoclinic sulphur.
-Plastic sulphur: - It is formed by boiling ordinary roll sulphur in a test tube and pouring the boiling mass into cold water. As a result, rubber like plastic sulphur is formed.
-Colloidal sulphur: - It is obtained by passing hydrogen sulphide through a solution of some oxidizing agents like nitric acid, potassium permanganate, sulphur dioxide solution etc.
\[2HN{O_3} + {H_2}S \to 2N{O_2} + 2{H_2}O + S \downarrow \]
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




