
The number of structural isomers of ${C_5}{H_{10}}$ is :-
A. 10
B. 11
C. 12
D. 13
Answer
566.7k+ views
Hint: The given compound does not have any groups and only hydrocarbons. It can form only chain structural isomers and ring structural isomers. Draw all possible isomers and count them.
Complete step by step answer:
Structural isomers have further categories as chain, positional, functional, tautomerism, metamerism and ring-chain. We have been given a formula as ${C_5}{H_{10}}$ with no other group of elements other than hydrogen and carbon. So only two types of structural isomers are possible. Chain isomerism and ring-chain isomerism. We will draw all possible isomers and count them. As the formula is of the form ${C_n}{H_{2n}}$ then the structural isomers are either alkenes or ring compounds. They are as follows starting with chain compounds and then ring compounds:
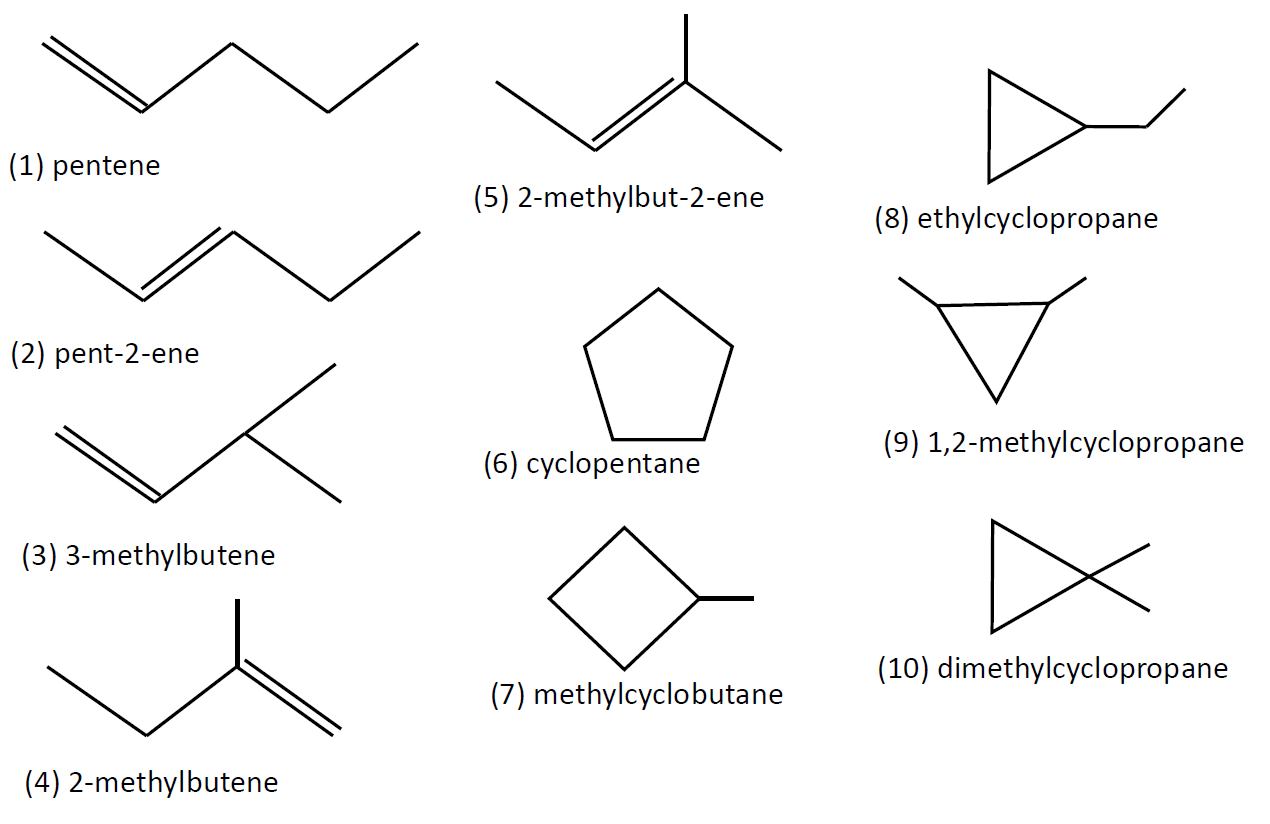
In the above set of diagrams, we formed ten isomers falling in structural isomers categories.
So, the correct answer is “Option A”.
Note: There is no standard way to calculate the number of structural isomers. Although, it will be laborious work to form an expression separately for each example using permutations and combinations of how groups, hydrogen atoms can be arranged with the carbon atoms.
Complete step by step answer:
Structural isomers have further categories as chain, positional, functional, tautomerism, metamerism and ring-chain. We have been given a formula as ${C_5}{H_{10}}$ with no other group of elements other than hydrogen and carbon. So only two types of structural isomers are possible. Chain isomerism and ring-chain isomerism. We will draw all possible isomers and count them. As the formula is of the form ${C_n}{H_{2n}}$ then the structural isomers are either alkenes or ring compounds. They are as follows starting with chain compounds and then ring compounds:

In the above set of diagrams, we formed ten isomers falling in structural isomers categories.
So, the correct answer is “Option A”.
Note: There is no standard way to calculate the number of structural isomers. Although, it will be laborious work to form an expression separately for each example using permutations and combinations of how groups, hydrogen atoms can be arranged with the carbon atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




