
The number of structural isomers possible with the formula ${C_4}{H_9}Cl$ are:
A. 5
B. 4
C. 3
D. 2
Answer
569.7k+ views
Hint: The structural isomers are defined as the organic compounds which have similar molecular formula but differ in their structural arrangement. The structural isomers are possible in hydrocarbons where more than two carbon atoms are present In ${C_4}{H_9}Cl$, four carbon atoms are present.
Complete step by step answer:
The isomers are defined as the molecules which possess the same molecular formulas but differ in the arrangement of atoms and groups. Isomers are divided into conformational isomers, structural isomers, stereoisomers, geometric isomers, optical isomers.
The structural isomers are defined as the organic compounds which have similar molecular formula but differ in their structural arrangement.
In haloalkanes, the number of carbon atoms, hydrogen atom and halogen atom are the same but the arrangement of carbon atoms, hydrogen atoms and halogen atoms are different.
The chemical formula given is ${C_4}{H_9}Cl$, where four carbon atoms are present, nine hydrogen atoms are present and one chlorine atom is present.
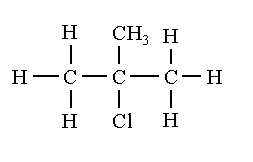
To draw the structural isomers of ${C_4}{H_9}Cl$, first draw a straight chain butane hydrocarbon and replace one hydrogen with the chlorine atom to form 1-chloro-2-methylpropane and 2-chloro-2methyl methylpropane. Secondly draw three
The structural isomers of ${C_4}{H_9}Cl$ are shown below.
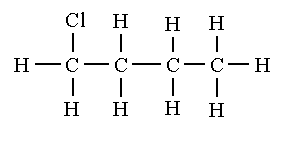
1-chlorobutane
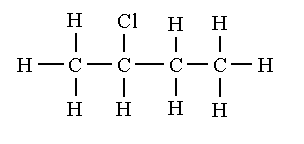
2-chlorobutane
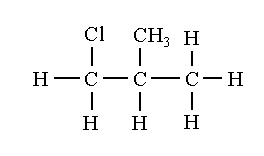
1-chloro-2-methylpropane
So, the correct answer is “Option B”.
Note:
The structural isomers are also called constitutional isomers. The structural isomers of alkyl halide are all different compounds therefore, they all possess different physical and chemical properties.
Complete step by step answer:
The isomers are defined as the molecules which possess the same molecular formulas but differ in the arrangement of atoms and groups. Isomers are divided into conformational isomers, structural isomers, stereoisomers, geometric isomers, optical isomers.
The structural isomers are defined as the organic compounds which have similar molecular formula but differ in their structural arrangement.
In haloalkanes, the number of carbon atoms, hydrogen atom and halogen atom are the same but the arrangement of carbon atoms, hydrogen atoms and halogen atoms are different.
The chemical formula given is ${C_4}{H_9}Cl$, where four carbon atoms are present, nine hydrogen atoms are present and one chlorine atom is present.
To draw the structural isomers of ${C_4}{H_9}Cl$, first draw a straight chain butane hydrocarbon and replace one hydrogen with the chlorine atom to form 1-chloro-2-methylpropane and 2-chloro-2methyl methylpropane. Secondly draw three
The structural isomers of ${C_4}{H_9}Cl$ are shown below.
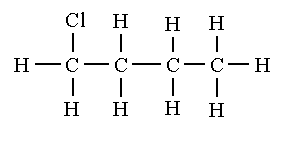
1-chlorobutane

2-chlorobutane

1-chloro-2-methylpropane

So, the correct answer is “Option B”.
Note:
The structural isomers are also called constitutional isomers. The structural isomers of alkyl halide are all different compounds therefore, they all possess different physical and chemical properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




