
The octet rule is not valid for which of the following molecules?
(A) $C{O_2}$
(B) ${H_2}O$
(C) ${O_2}$
(D) $NO$
Answer
569.4k+ views
Hint: As we know that octet rule involves the atoms of various elements that tend to gain, lose or share the valence electrons during the formation of molecules such that there are eight electrons or octet in their valence shells and similar atoms share the electrons by bond formation through sharing of electrons.
Complete Step by step answer:As we know that octet rule involves the atoms that gain, lose or share their valence electrons and form a molecule such that there are eight electrons in their valence shells. The gain or loss of electrons cannot take place between similar atoms, in such cases bond formation takes place by mutual sharing of electrons which is called covalent bond.
Let us talk about each molecule to understand better.
We know that carbon possesses four electrons in its valence shell so to attain the octet or eight electrons it shares its electrons with oxygen which possesses six electrons in its valence shell. So two oxygen molecules share two electrons each with four electrons of carbon which we can show as following:
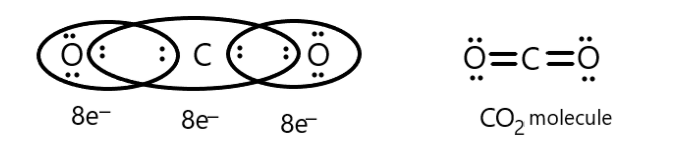
Thus, carbon dioxide follows octet rule.
Now comes the water molecule, we know that hydrogen atoms contain one electron in its valence shell and attain its duplet by sharing these electrons with oxygen possessing six valence electrons. We can show it as the following:
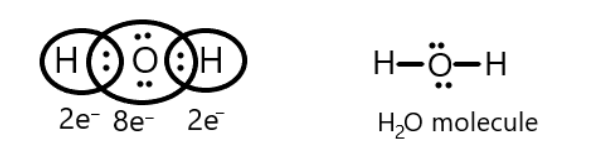
Thus, it is showing the octet rule is followed by water molecules.
Similarly, we can show the octet of oxygen molecules that possess similar atoms and thus share only two electrons each to complete their octet. This can be shown as:
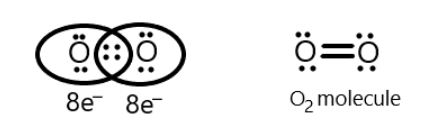
Thus oxygen molecules also obey octet rule.
But in case of nitrogen we know that it contains five electrons in its valence shell and oxygen possesses six electrons in its valence resulting in an odd electron molecule, thus the octet rule is not followed in the $NO$ molecule.
Hence the correct answer is (D).
Note: Octet rule is responsible for better understanding of the structure of most of the organic compounds and thus it is applicable to mainly second period elements of the periodic table but it is not a universal rule to because some structures are not explained using octet rule like the structure of $Be{F_2}$etc.
Complete Step by step answer:As we know that octet rule involves the atoms that gain, lose or share their valence electrons and form a molecule such that there are eight electrons in their valence shells. The gain or loss of electrons cannot take place between similar atoms, in such cases bond formation takes place by mutual sharing of electrons which is called covalent bond.
Let us talk about each molecule to understand better.
We know that carbon possesses four electrons in its valence shell so to attain the octet or eight electrons it shares its electrons with oxygen which possesses six electrons in its valence shell. So two oxygen molecules share two electrons each with four electrons of carbon which we can show as following:

Thus, carbon dioxide follows octet rule.
Now comes the water molecule, we know that hydrogen atoms contain one electron in its valence shell and attain its duplet by sharing these electrons with oxygen possessing six valence electrons. We can show it as the following:

Thus, it is showing the octet rule is followed by water molecules.
Similarly, we can show the octet of oxygen molecules that possess similar atoms and thus share only two electrons each to complete their octet. This can be shown as:

Thus oxygen molecules also obey octet rule.
But in case of nitrogen we know that it contains five electrons in its valence shell and oxygen possesses six electrons in its valence resulting in an odd electron molecule, thus the octet rule is not followed in the $NO$ molecule.
Hence the correct answer is (D).
Note: Octet rule is responsible for better understanding of the structure of most of the organic compounds and thus it is applicable to mainly second period elements of the periodic table but it is not a universal rule to because some structures are not explained using octet rule like the structure of $Be{F_2}$etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




