
The orbital overlapping is maximum in:
A.$C{l_2}$
B.HI
C.HCl
D.HBr
Answer
589.2k+ views
Hint: In chemical bonds, an orbital overlap is the concentration of orbitals on adjacent atoms in the same regions of space. Pi bond occurs when two orbitals overlap outside the space between the nuclei (I.E. outside the internuclear axis).
Complete step by step answer:
-The atoms combine by colliding with each other. This situation refers to the process in which the two atoms come so close to each other that they penetrate each other’s orbital anf form a new hybridized orbital where the bonding pair of electrons reside. This hybridized orbital has lower energy than the atomic orbital and hence is stable.
-Therefore, this partial penetration of orbital is known as orbital overlap. The greater the overlap, the stronger is the bond formation.
-Now, the mutual overlap between two half-filled p-orbitals of two atoms is known as p-p overlapping. So, the covalent bond formed is called a p-p bond.
-Among the given options, the orbital overlapping is maximum in case of $C{l_2}$. This is because p-p overlapping is more effective than s-p overlapping which is more effective than s-s overlapping.
-In case of $C{l_2}$ molecules, the bond is formed by p-p overlapping whereas in case of HI, HCl and HBr, there is s-p overlapping.
The different types of overlapping is as shown:
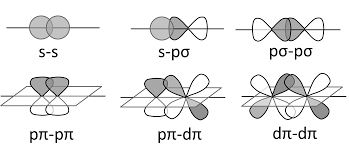
Hence, option A is correct.
Note:
It is important to note that the head-to-head overlapping of two p orbitals gives a sigma bond whereas the lateral overlap of these orbitals leads to the formation of pi-bonds. The pi-bonds are generally weaker than sigma bonds and the combination of sigma and pi bonds is always stronger than a single sigma bond.
Complete step by step answer:
-The atoms combine by colliding with each other. This situation refers to the process in which the two atoms come so close to each other that they penetrate each other’s orbital anf form a new hybridized orbital where the bonding pair of electrons reside. This hybridized orbital has lower energy than the atomic orbital and hence is stable.
-Therefore, this partial penetration of orbital is known as orbital overlap. The greater the overlap, the stronger is the bond formation.
-Now, the mutual overlap between two half-filled p-orbitals of two atoms is known as p-p overlapping. So, the covalent bond formed is called a p-p bond.
-Among the given options, the orbital overlapping is maximum in case of $C{l_2}$. This is because p-p overlapping is more effective than s-p overlapping which is more effective than s-s overlapping.
-In case of $C{l_2}$ molecules, the bond is formed by p-p overlapping whereas in case of HI, HCl and HBr, there is s-p overlapping.
The different types of overlapping is as shown:

Hence, option A is correct.
Note:
It is important to note that the head-to-head overlapping of two p orbitals gives a sigma bond whereas the lateral overlap of these orbitals leads to the formation of pi-bonds. The pi-bonds are generally weaker than sigma bonds and the combination of sigma and pi bonds is always stronger than a single sigma bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




