
The outer electron configuration of Gd (Atomic No. 64) is:
a.) \[4{{f}^{3}}5{{d}^{5}}6{{s}^{2}}\]
b.) \[4{{f}^{4}}5{{d}^{0}}6{{s}^{2}}\]
c.) \[4{{f}^{2}}5{{d}^{4}}6{{s}^{2}}\]
d.) \[4{{f}^{7}}5{{d}^{1}}6{{s}^{2}}\]
Answer
590.7k+ views
Hint: Lanthanides are also called ad f-block elements. The common electronic configuration of lanthanides is \[4{{f}^{1-14}}5{{d}^{0-1}}6{{s}^{2}}\]. Sometimes the electrons may enter into 5d orbital to get a full filled or half-filled electron configuration in 4f orbital.
Complete step by step answer:
We know that Gadolinium is a chemical element with the symbol Gd belonging to f-block.
The atomic number of gadolinium is 64.
It is a silvery-white, soft, ductile metal.
Gadolinium is found in nature only in combined form with other chemical elements.
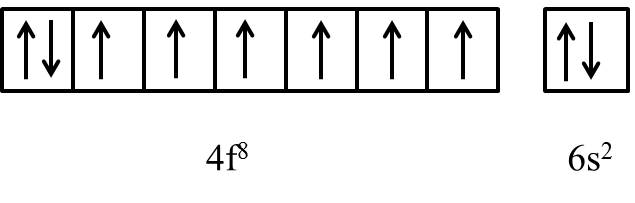
The general electronic configuration of Gd is [Xe]\[4{{f}^{8}}5{{d}^{0}}6{{s}^{2}}\].
As per above electronic configuration in f-orbital first seven electrons occupies all the seven orbitals and the 8th electrons starts pairing with any one of the electrons in 7 degenerate orbitals of 4f orbital.
We know that if any element has half or fully filled electrons in their outermost orbital then the element is more stable.
Due to the above reason f-block elements like Gadolinium shows an half-filled electronic configuration by sending the 8th electron in 4f orbital to 5d orbital and looks like as follows.

Therefore, the outer electronic configuration of Gadolinium metal is [Xe]\[4{{f}^{7}}5{{d}^{1}}6{{s}^{2}}\].
So, the correct answer is “Option D”.
Note: Electronic configuration of Gadolinium metal is [Xe] \[4{{f}^{7}}5{{d}^{1}}6{{s}^{2}}\] instead of [Xe]\[4{{f}^{8}}5{{d}^{0}}6{{s}^{2}}\].
This half-filled electronic configuration of 4f-orbital makes Gadolinium metal as exceptionally stable.
Gadolinium loses three electrons very easily, 2 electrons from 6s orbital and one electron from 5d orbital. The stable oxidation state of gadolinium is +3.
Complete step by step answer:
We know that Gadolinium is a chemical element with the symbol Gd belonging to f-block.
The atomic number of gadolinium is 64.
It is a silvery-white, soft, ductile metal.
Gadolinium is found in nature only in combined form with other chemical elements.
The general electronic configuration of Gd is [Xe]\[4{{f}^{8}}5{{d}^{0}}6{{s}^{2}}\].
As per above electronic configuration in f-orbital first seven electrons occupies all the seven orbitals and the 8th electrons starts pairing with any one of the electrons in 7 degenerate orbitals of 4f orbital.

We know that if any element has half or fully filled electrons in their outermost orbital then the element is more stable.
Due to the above reason f-block elements like Gadolinium shows an half-filled electronic configuration by sending the 8th electron in 4f orbital to 5d orbital and looks like as follows.

Therefore, the outer electronic configuration of Gadolinium metal is [Xe]\[4{{f}^{7}}5{{d}^{1}}6{{s}^{2}}\].
So, the correct answer is “Option D”.
Note: Electronic configuration of Gadolinium metal is [Xe] \[4{{f}^{7}}5{{d}^{1}}6{{s}^{2}}\] instead of [Xe]\[4{{f}^{8}}5{{d}^{0}}6{{s}^{2}}\].
This half-filled electronic configuration of 4f-orbital makes Gadolinium metal as exceptionally stable.
Gadolinium loses three electrons very easily, 2 electrons from 6s orbital and one electron from 5d orbital. The stable oxidation state of gadolinium is +3.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




