
The oxidation of toluene to benzaldehyde by chromyl chloride is called:
A. Etard reaction
B. Riemer-Tiemann reaction
C. Wurtz reaction
D. Cannizzaro’s reaction
Answer
585.6k+ views
Hint: Benzaldehyde is an important organic compound consisting of a benzene ring with formyl (CHO) group which is mainly used in the food industry. Chromyl chloride behaves as a weak acid in a nonpolar solvent. The methyl group of toluene gets partially oxidized in this reaction to form benzaldehyde. Check the options and find out which of the name-reactions uses chromyl chloride as the reagent.
Complete step by step answer:
The conversion of toluene $\left( {{C}_{6}}{{H}_{5}}C{{H}_{3}} \right)$ to benzaldehyde $\left( {{C}_{6}}{{H}_{5}}CHO \right)$ using chromyl chloride $\left( Cr{{O}_{2}}C{{l}_{2}} \right)$ is an oxidation reaction and this reaction is also known as Etard reaction.
Etard reaction is a very important reaction for the conversion of toluene to benzaldehyde. In this reaction, a compound which has at least one methyl group bonded to the benzene ring is required and when this methyl group is oxidised then it changes to an aldehyde. It is done by using a weak oxidizing agent, therefore, chromyl chloride is used since it behaves as a weak oxidizing agent in a nonpolar solvent.
The overall reaction mechanism is shown below:
Step I: The chromyl chloride abstracts hydrogen from the methyl group in toluene and forms a bond with the carbon of the phenyl ring forming a precipitate known as Etard complex.
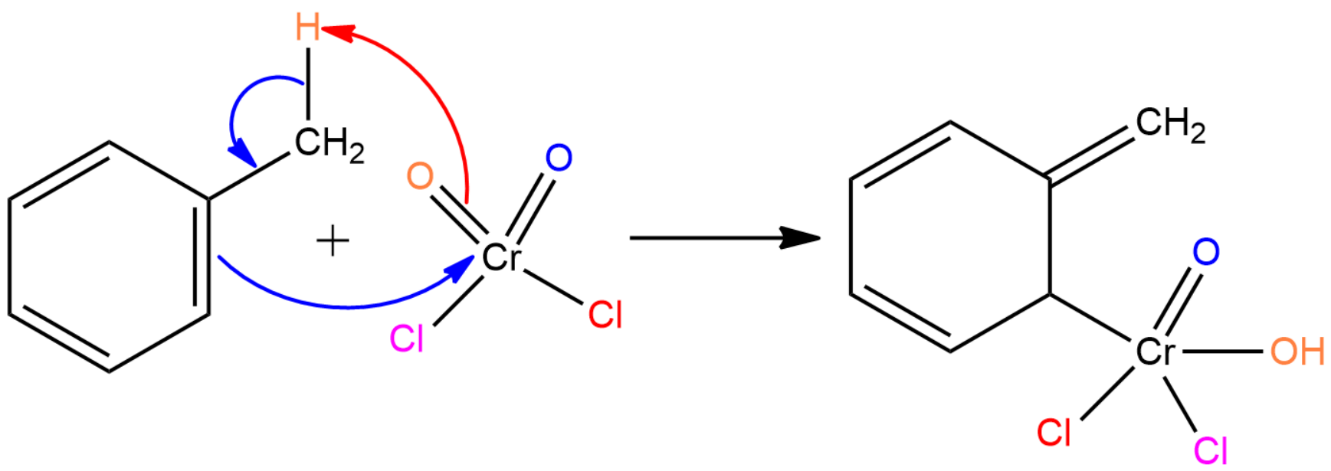
Step II: The Etard complex undergoes a [2,3] sigmatropic rearrangement and forms a rearrangement product.
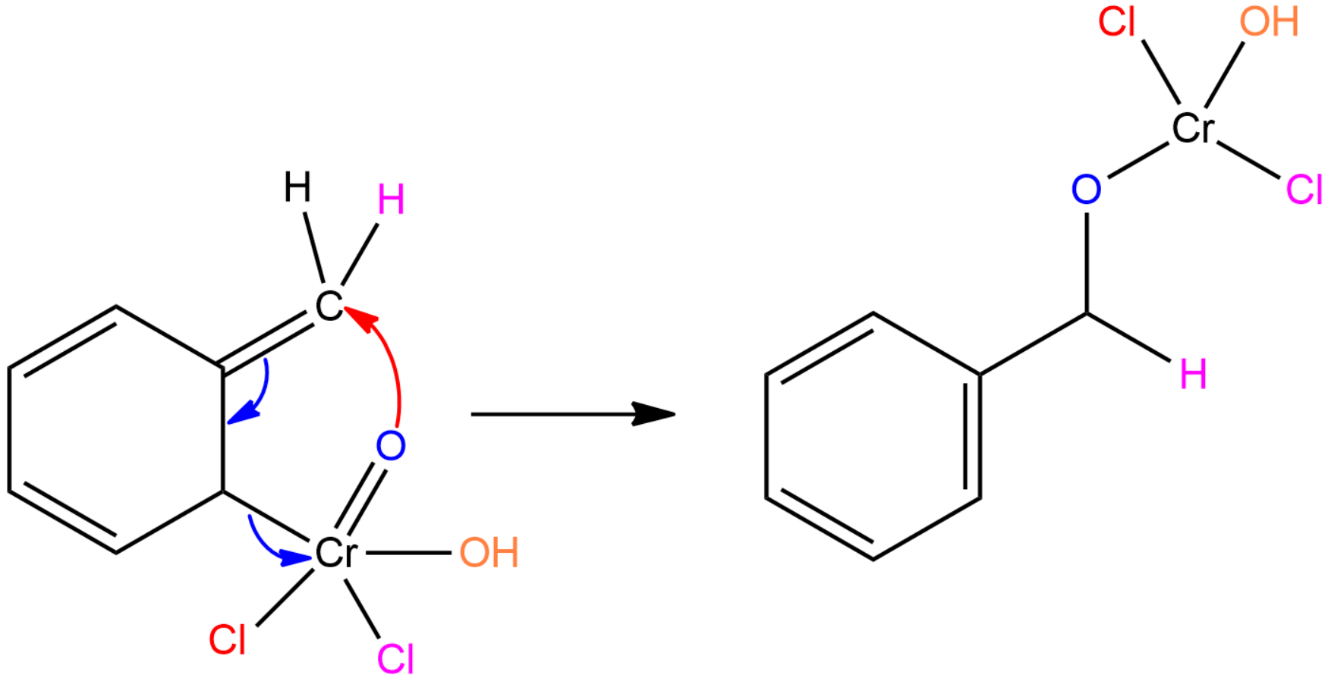
Step III: Cr(Cl)OH and HCl comes out from the rearrangement product to form benzaldehyde.
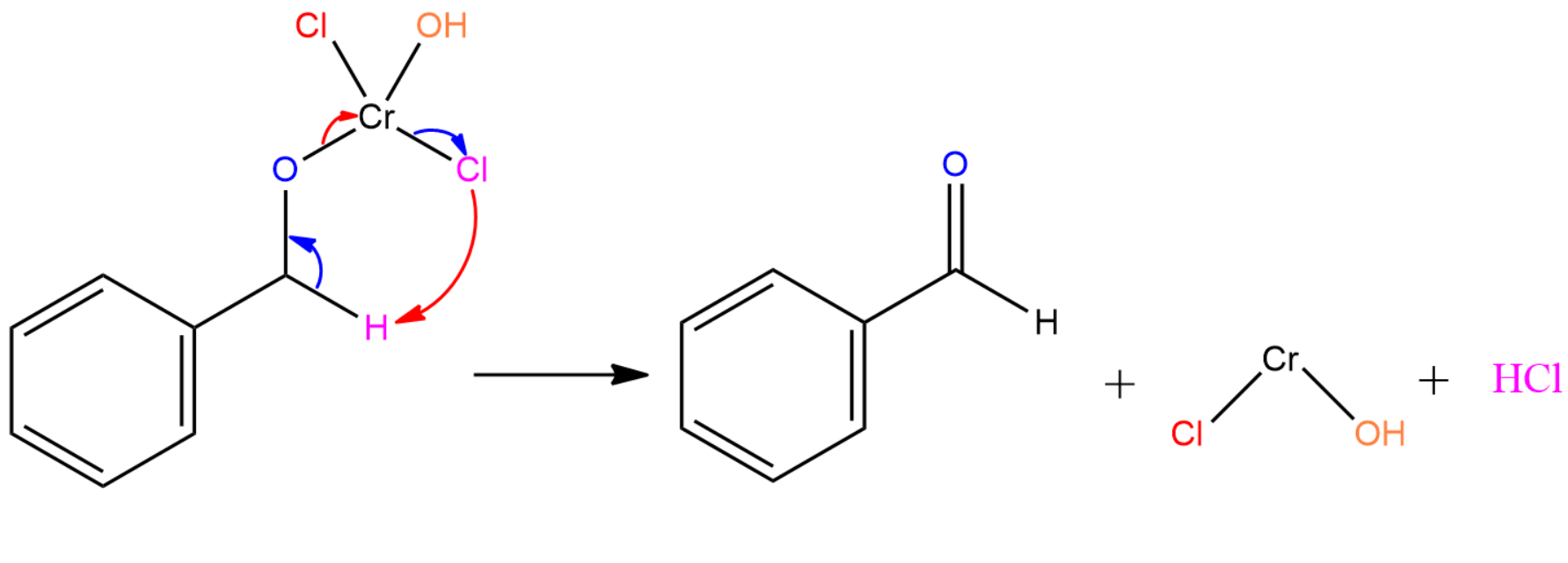
Hence, option (A) is the correct one.
Additional information:
- The Riemer-Tiemann reaction is the reactions which are used for the preparation of salicylaldehyde when phenol is treated with chloroform in the presence of bases like KOH.
- Wurtz reaction is used to prepare higher alkane i.e. alkane with one extra carbon from lower alkane i.e. alkane with one less carbon by using alkyl halide with sodium in dry ether.
- In Cannizaro’s reaction, aldehyde which does not have alpha hydrogen atoms undergo self-oxidation and reduction reaction on heating with concentrated alkali. In this reaction, one molecule of aldehyde is reduced to alcohol while another is oxidised to a carboxylic acid.
- Thus other options are incorrect.
Note: In this reaction, carbon tetrachloride is used as a solvent since it is the most common non-polar solvent. As we know that chromyl chloride is a mild oxidizing agent, it is the perfect reagent to convert toluene to aldehyde. This is because a strong oxidizing agent will further oxidize the benzaldehyde formed to benzoic acid.
Complete step by step answer:
The conversion of toluene $\left( {{C}_{6}}{{H}_{5}}C{{H}_{3}} \right)$ to benzaldehyde $\left( {{C}_{6}}{{H}_{5}}CHO \right)$ using chromyl chloride $\left( Cr{{O}_{2}}C{{l}_{2}} \right)$ is an oxidation reaction and this reaction is also known as Etard reaction.
Etard reaction is a very important reaction for the conversion of toluene to benzaldehyde. In this reaction, a compound which has at least one methyl group bonded to the benzene ring is required and when this methyl group is oxidised then it changes to an aldehyde. It is done by using a weak oxidizing agent, therefore, chromyl chloride is used since it behaves as a weak oxidizing agent in a nonpolar solvent.
The overall reaction mechanism is shown below:
Step I: The chromyl chloride abstracts hydrogen from the methyl group in toluene and forms a bond with the carbon of the phenyl ring forming a precipitate known as Etard complex.

Step II: The Etard complex undergoes a [2,3] sigmatropic rearrangement and forms a rearrangement product.

Step III: Cr(Cl)OH and HCl comes out from the rearrangement product to form benzaldehyde.

Hence, option (A) is the correct one.
Additional information:
- The Riemer-Tiemann reaction is the reactions which are used for the preparation of salicylaldehyde when phenol is treated with chloroform in the presence of bases like KOH.
- Wurtz reaction is used to prepare higher alkane i.e. alkane with one extra carbon from lower alkane i.e. alkane with one less carbon by using alkyl halide with sodium in dry ether.
- In Cannizaro’s reaction, aldehyde which does not have alpha hydrogen atoms undergo self-oxidation and reduction reaction on heating with concentrated alkali. In this reaction, one molecule of aldehyde is reduced to alcohol while another is oxidised to a carboxylic acid.
- Thus other options are incorrect.
Note: In this reaction, carbon tetrachloride is used as a solvent since it is the most common non-polar solvent. As we know that chromyl chloride is a mild oxidizing agent, it is the perfect reagent to convert toluene to aldehyde. This is because a strong oxidizing agent will further oxidize the benzaldehyde formed to benzoic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




