
The oxide of nitrogen which has three-electron bond in its structure is
A.\[{N_2}O\]
B.$NO$
C.${N_2}{O_3}$
D.${N_2}{O_5}$
Answer
564.9k+ views
Hint:For an electron to possess three electron bonds it should have bond multiplicity $1.5$ , that means the molecule should contain one unpaired electron in it. This question’s answer can be obtained by drawing its Lewis structure or by simply its structure showing the non-bonding electrons.
Complete step by step answer:
Let us first learn about the different oxides of nitrogen;
Nitrogen oxides are a combination of nitrogen and oxygen-composed gases. There are many oxides of nitrogen among which nitric oxide $\,(NO)\,$ is the most toxic one.
Now, let us understand a three-electron bond;
Three- electron bond is a virtual assumption which says that a bond is formed by three electrons with its relative spins. For a molecule to possess three electron- bond its multiplicity should be $1.5$.
Let’s now analyze the structure of each element given in the options;
A.${N_2}O$
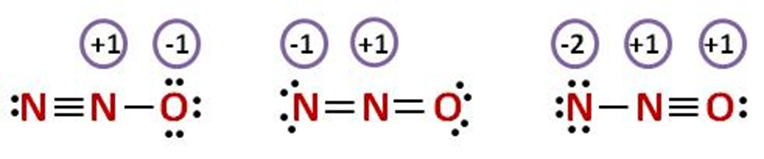
No unpaired electrons are there in this structure to form a three – electron bond since octet is complete for every nitrogen and oxygen atom in this structure.
B.$NO$
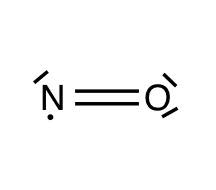
Here one unpaired electron is there. So, in this structure, the nitrogen possesses only seven electrons and the octet is not completed. So, the one oxygen will donate its one electron to form a three- electron bond (means one single bond has three electrons) which makes the octet of nitrogen complete.
So, this is our correct answer to this question.
C.$\,{N_2}{O_3}\,$
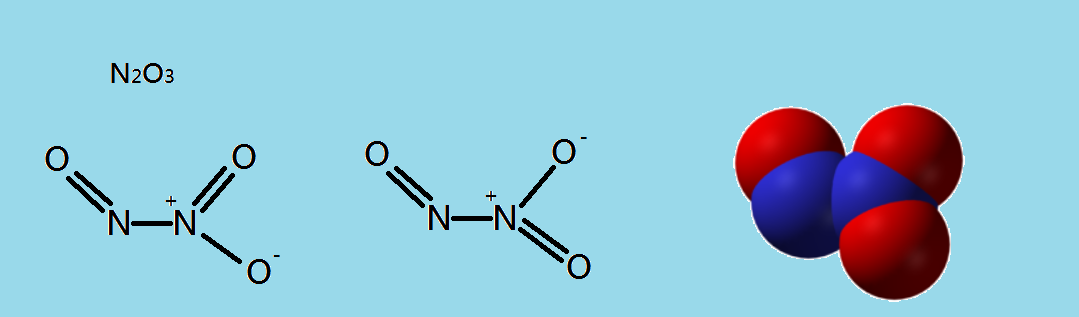
In the structure of \[{N_2}{O_3}\] there is one single bond between two $N$ atom and one single bond between $N$ and $O$ atom, and two double bonds are there in between $N$ and $O$ atom. So there are no unpaired electrons and the nitrogen and oxygen atom’s octet is complete so no three- electron bond formation occurs.
D.$\,{N_2}{O_5}\,$

In ${N_2}{O_5}$ there are four $N - O$ single bonds and two $N = O$ double bonds. And no unpaired electrons and so the octet of nitrogen is complete and hence, no formation of three- electron bond.
So, the correct answer to this question is option B that is $NO$ .
Additional information:
Multiplicity is the number of possible orientations of spin in an energy level or the number of electron pairs in the region when two atoms bonded together. Each electron possesses a spin multiplicity$ + \dfrac{1}{2}$ or $ - \dfrac{1}{2}$ .
Three electron bonds deviate from Pauli’s exclusion principle which says that one orbital can only have two electrons maximum. So, it is just only an assumption for the octet completion.
Note: Note that here the multiplicity means that when nitrogen is bonded to oxygen with $3$ bonds, one bond can be formed by two electrons from oxygen and one electron from nitrogen so there will be three spins possible in a single bond, it is only a virtual assumption.
Complete step by step answer:
Let us first learn about the different oxides of nitrogen;
Nitrogen oxides are a combination of nitrogen and oxygen-composed gases. There are many oxides of nitrogen among which nitric oxide $\,(NO)\,$ is the most toxic one.
Now, let us understand a three-electron bond;
Three- electron bond is a virtual assumption which says that a bond is formed by three electrons with its relative spins. For a molecule to possess three electron- bond its multiplicity should be $1.5$.
Let’s now analyze the structure of each element given in the options;
A.${N_2}O$
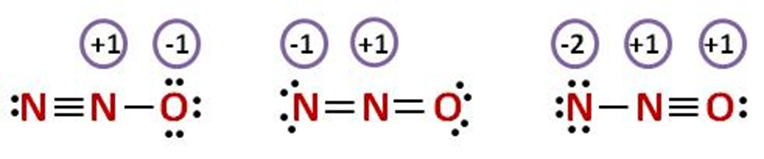
No unpaired electrons are there in this structure to form a three – electron bond since octet is complete for every nitrogen and oxygen atom in this structure.
B.$NO$

Here one unpaired electron is there. So, in this structure, the nitrogen possesses only seven electrons and the octet is not completed. So, the one oxygen will donate its one electron to form a three- electron bond (means one single bond has three electrons) which makes the octet of nitrogen complete.
So, this is our correct answer to this question.
C.$\,{N_2}{O_3}\,$

In the structure of \[{N_2}{O_3}\] there is one single bond between two $N$ atom and one single bond between $N$ and $O$ atom, and two double bonds are there in between $N$ and $O$ atom. So there are no unpaired electrons and the nitrogen and oxygen atom’s octet is complete so no three- electron bond formation occurs.
D.$\,{N_2}{O_5}\,$

In ${N_2}{O_5}$ there are four $N - O$ single bonds and two $N = O$ double bonds. And no unpaired electrons and so the octet of nitrogen is complete and hence, no formation of three- electron bond.
So, the correct answer to this question is option B that is $NO$ .
Additional information:
Multiplicity is the number of possible orientations of spin in an energy level or the number of electron pairs in the region when two atoms bonded together. Each electron possesses a spin multiplicity$ + \dfrac{1}{2}$ or $ - \dfrac{1}{2}$ .
Three electron bonds deviate from Pauli’s exclusion principle which says that one orbital can only have two electrons maximum. So, it is just only an assumption for the octet completion.
Note: Note that here the multiplicity means that when nitrogen is bonded to oxygen with $3$ bonds, one bond can be formed by two electrons from oxygen and one electron from nitrogen so there will be three spins possible in a single bond, it is only a virtual assumption.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




