
The $P - P - P$ in ${P_4}$ molecule and $S - S - S$ angle in ${S_8}$ molecule is (in degree) respectively:
(A) ${60^\circ },{107^\circ }$
(B) ${107^\circ },{60^\circ }$
(C) ${60^\circ },{108^\circ }$
(D) ${60^\circ },{40^\circ }$
Answer
568.5k+ views
Hint: Atoms in ${P_4}$ molecules are arranged in a tetrahedron structure. Whereas ${S_8}$ molecules’ atoms are arranged in a crown shape. Thus, they will have different angles between their atoms corresponding to their respective structure.
Complete step by step answer:
${P_4}$ (tetra phosphorus) is a member of tetratomic phosphorus.
A nonmetal element that has the atomic symbol P, atomic number $15$ and atomic mass $31$. It is an essential element that takes part in a broad variety of biochemical reactions.
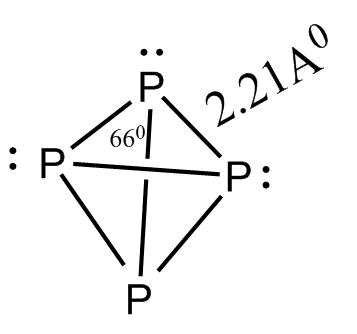
In ${P_4}$ molecule the four $S{p^3}$ hybridized phosphorus atoms lie at the corners of a regular tetrahedron with $P - P - P$ angle ${60^\circ }$
$P_4$ has following characteristics:
-Tetrahedron shape
-P-P-P bond angle is ${60^\circ }$
-There are six bonds in total.
-Four lone pairs of electrons.
-Can form a single bond with other atoms ${S_8}$ molecules.
Cyclo octa Sulphur is a homo monocyclic compound composed of eight Sulphur atoms. It has been isolated from Ganoderma lucidum, a mushroom commonly used in Chinese medicine. It has a role as a fungal metabolic and a bacterial metabolite. It is an elemental Sulphur and a homo monocyclic compound.
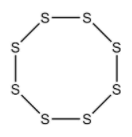
Top View
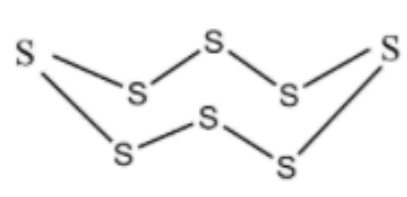
Side View
In ${S_8}$ molecule, $S - S - S$ angle is ${107^\circ }$.
So, the answer is (A) ${60^\circ },{107^\circ }$.
Note: The Molecular solid is a solid consisting of discrete molecules. The cohesive forces are Van Der waal forces, dipole-dipole interaction, etc. So ${P_4}$ and ${S_8}$ are called molecular solids.
Complete step by step answer:
${P_4}$ (tetra phosphorus) is a member of tetratomic phosphorus.
A nonmetal element that has the atomic symbol P, atomic number $15$ and atomic mass $31$. It is an essential element that takes part in a broad variety of biochemical reactions.

In ${P_4}$ molecule the four $S{p^3}$ hybridized phosphorus atoms lie at the corners of a regular tetrahedron with $P - P - P$ angle ${60^\circ }$
$P_4$ has following characteristics:
-Tetrahedron shape
-P-P-P bond angle is ${60^\circ }$
-There are six bonds in total.
-Four lone pairs of electrons.
-Can form a single bond with other atoms ${S_8}$ molecules.
Cyclo octa Sulphur is a homo monocyclic compound composed of eight Sulphur atoms. It has been isolated from Ganoderma lucidum, a mushroom commonly used in Chinese medicine. It has a role as a fungal metabolic and a bacterial metabolite. It is an elemental Sulphur and a homo monocyclic compound.

Top View

Side View
In ${S_8}$ molecule, $S - S - S$ angle is ${107^\circ }$.
So, the answer is (A) ${60^\circ },{107^\circ }$.
Note: The Molecular solid is a solid consisting of discrete molecules. The cohesive forces are Van Der waal forces, dipole-dipole interaction, etc. So ${P_4}$ and ${S_8}$ are called molecular solids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




