
The population inversions in helium – laser is produced by?
A) Photon excitation
B) Chemical reaction
C) Inelastic atomic collision
D) Electron excitation
Answer
584.1k+ views
Hint:Population inversion is the state in which the number of atoms in higher energy state is more than those in lower energy state. Population inversion is the process of achieving more electrons in the higher energy state as compared to the lower energy state
This state is achieved by the pumping by high DC supply in He-Ne lasers.
Step by step solution:
In general, the lower energy state has more electrons than the higher energy state. However, after achieving population inversion, more electrons will remain in the higher energy state than the lower energy state.
As shown in diagram of energy state
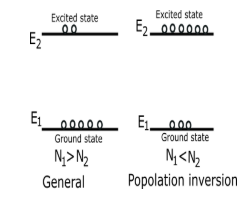
${E_1}$ Is the ground state
${E_2}$ Is the excited state or higher state than ground state
General situation ${N_1}\rangle {N_2}$
In population inversion ${N_1}\langle {N_2}$
To achieve this state we have to supply some kind of energy to the Atom at ground state.
In helium neon lasers we use a high voltage DC Power supply as a pump source. A high voltage DC supply electric current throws the gas mixture of helium and neon.
When the high energetic electron goes through the gas mixture then it collides with the helium atom and excited helium. These helium atoms Collide with ground states ( ${E_1}$ ) neon atoms and excite them to higher energy states ( ${E_3}$ ). At this state atom can not stay for long time they emits some energy and go to the metastable state ( ${E_2}$ ) here electron can stay for long time comparison to excited state so the no of electron become higher than the ground state( ${N_1}\langle {N_2}$ ) this produce population inversion.
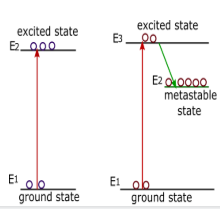
This process is known as electron excitation which is normally used in gas lasers.
Hence in this question the option D is correct
Note:Different types of lasers use different types of methods to achieve population inversion. As A ruby laser most often consists of a ruby rod that must be pumped with very high energy, usually from a flashtube, to achieve a population inversion. Population inversion is important to achieve laser action.
Helium neon laser produces a continuous laser beam of red colour having wavelength 6328 Angstrom.
This state is achieved by the pumping by high DC supply in He-Ne lasers.
Step by step solution:
In general, the lower energy state has more electrons than the higher energy state. However, after achieving population inversion, more electrons will remain in the higher energy state than the lower energy state.
As shown in diagram of energy state

${E_1}$ Is the ground state
${E_2}$ Is the excited state or higher state than ground state
General situation ${N_1}\rangle {N_2}$
In population inversion ${N_1}\langle {N_2}$
To achieve this state we have to supply some kind of energy to the Atom at ground state.
In helium neon lasers we use a high voltage DC Power supply as a pump source. A high voltage DC supply electric current throws the gas mixture of helium and neon.
When the high energetic electron goes through the gas mixture then it collides with the helium atom and excited helium. These helium atoms Collide with ground states ( ${E_1}$ ) neon atoms and excite them to higher energy states ( ${E_3}$ ). At this state atom can not stay for long time they emits some energy and go to the metastable state ( ${E_2}$ ) here electron can stay for long time comparison to excited state so the no of electron become higher than the ground state( ${N_1}\langle {N_2}$ ) this produce population inversion.

This process is known as electron excitation which is normally used in gas lasers.
Hence in this question the option D is correct
Note:Different types of lasers use different types of methods to achieve population inversion. As A ruby laser most often consists of a ruby rod that must be pumped with very high energy, usually from a flashtube, to achieve a population inversion. Population inversion is important to achieve laser action.
Helium neon laser produces a continuous laser beam of red colour having wavelength 6328 Angstrom.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




