
The pyrimidine bases present in DNA are:
A. cytosine and adenine
B. cytosine and guanine
C. cytosine and thymine
D. cytosine and uracil
Answer
594k+ views
Hint: A pyrimidine is a six-membered nitrogenous heterocyclic compound. Pyrimidine bases are weak bases. They are stabilized by resonance in the ring, due to which there is partial double bond character. Substituted pyrimidines are components of (RNA) ribonucleic acids and (DNA) deoxyribonucleic acids.
Complete answer:
Pyrimidines are aromatic nitrogen heterocycles with a structure similar to benzene but containing two nitrogen atoms at the 1 and 3 positions of the ring, with the molecular formula (${{\text{C}}_{4}}{{\text{H}}_{4}}{{\text{N}}_{2}}$). The structure of pyrimidine is
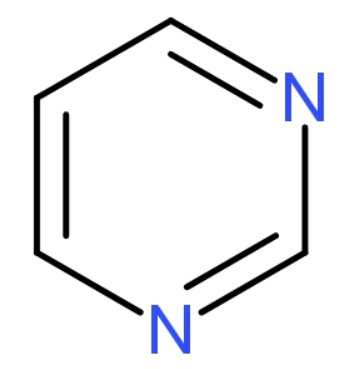
The pyrimidine bases present in DNA:
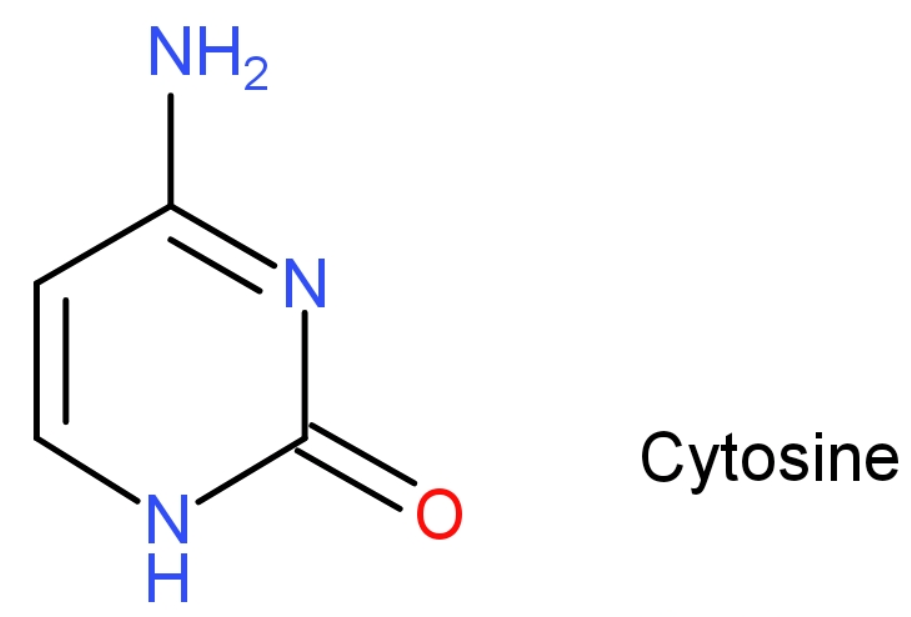
-Cytosine: Cytosine is a pyrimidine derivative base, with a heterocyclic, aromatic ring, with two substituents attached one, an amine group at positioned 4 and a keto group at positioned 2. It is present in both DNA and RNA. The molecular mass of cytosine is 111 grams. Structure of cytosine is
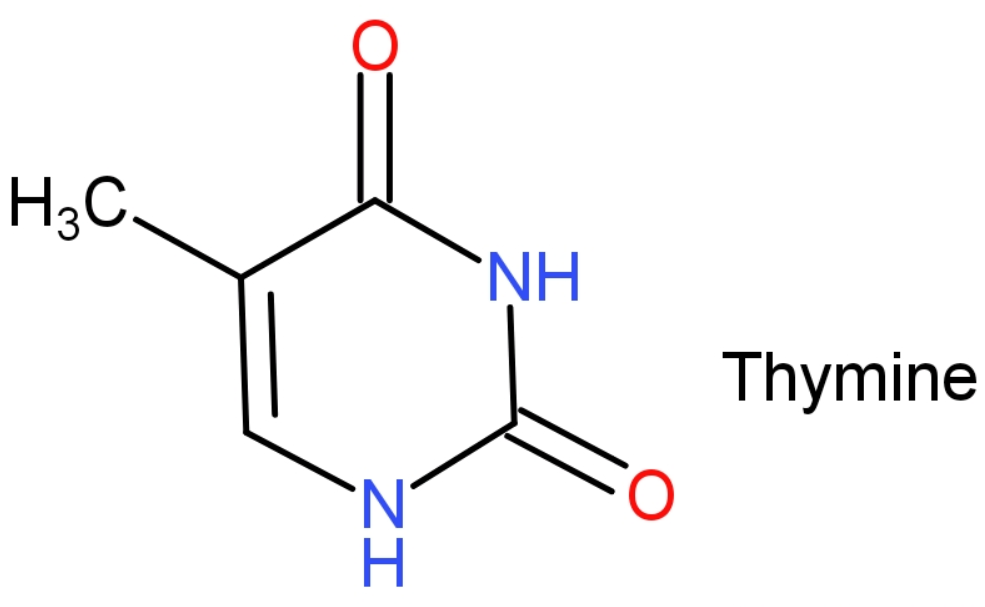
-Thymine: The IUPAC name of thymine is 5-methyl-1H-pyrimidine-2,4-dione. It is a pyrimidine nucleobase, which is present only in DNA. In DNA molecules, bases of Thymine are located on one strand form bonds with adenine bases on opposite strands. It is replaced by Uracil in RNA. The molecular mass of thymine is 126 grams. The structure of Thymine is
The purine bases include Adenine and Guanine whereas the pyrimidines include Thymine, Cytosine and Uracil.
A. cytosine and adenine: Cytosine is pyrimidine but Adenine is not a pyrimidine, hence it is incorrect.
B. cytosine and guanine: Cytosine is pyrimidine but Guanine is not a pyrimidine, hence it is incorrect.
D. cytosine and uracil: Cytosine is pyrimidine and Uracil is also a pyrimidine but it is not present in DNA, it is present in RNA, hence it is also incorrect.
So, the correct answer of the question is option ‘c’, which is Thymine and Cytosine.
So, the correct answer is “Option C”.
Additional Information: Uses of Cytosine:
(1) It acts as a cofactor to enzymes.
(2) It can transfer phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP).
Uses of Thymine:
(1) Thymine is vitamin ${{\text{B}}_{1}}$. Thymine is found in foods such as cereals, whole grains, nuts and beans. Thymine is important in breakdown of carbohydrates from foods into products required by the body.
(2) Thymine is used to treat or prevent vitamin ${{\text{B}}_{1}}$ deficiency. Thymine injection is used to treat beriberi, a condition caused by prolonged lack of vitamin ${{\text{B}}_{1}}$.
Note: The purines and pyrimidines bases are different. The purine bases include Adenine and Guanine whereas the pyrimidines include Thymine, Cytosine and Uracil. Thymine is in DNA. Thymine is replaced by uracil in RNA.
Complete answer:
Pyrimidines are aromatic nitrogen heterocycles with a structure similar to benzene but containing two nitrogen atoms at the 1 and 3 positions of the ring, with the molecular formula (${{\text{C}}_{4}}{{\text{H}}_{4}}{{\text{N}}_{2}}$). The structure of pyrimidine is

The pyrimidine bases present in DNA:
-Cytosine: Cytosine is a pyrimidine derivative base, with a heterocyclic, aromatic ring, with two substituents attached one, an amine group at positioned 4 and a keto group at positioned 2. It is present in both DNA and RNA. The molecular mass of cytosine is 111 grams. Structure of cytosine is

-Thymine: The IUPAC name of thymine is 5-methyl-1H-pyrimidine-2,4-dione. It is a pyrimidine nucleobase, which is present only in DNA. In DNA molecules, bases of Thymine are located on one strand form bonds with adenine bases on opposite strands. It is replaced by Uracil in RNA. The molecular mass of thymine is 126 grams. The structure of Thymine is

The purine bases include Adenine and Guanine whereas the pyrimidines include Thymine, Cytosine and Uracil.
A. cytosine and adenine: Cytosine is pyrimidine but Adenine is not a pyrimidine, hence it is incorrect.
B. cytosine and guanine: Cytosine is pyrimidine but Guanine is not a pyrimidine, hence it is incorrect.
D. cytosine and uracil: Cytosine is pyrimidine and Uracil is also a pyrimidine but it is not present in DNA, it is present in RNA, hence it is also incorrect.
So, the correct answer of the question is option ‘c’, which is Thymine and Cytosine.
So, the correct answer is “Option C”.
Additional Information: Uses of Cytosine:
(1) It acts as a cofactor to enzymes.
(2) It can transfer phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP).
Uses of Thymine:
(1) Thymine is vitamin ${{\text{B}}_{1}}$. Thymine is found in foods such as cereals, whole grains, nuts and beans. Thymine is important in breakdown of carbohydrates from foods into products required by the body.
(2) Thymine is used to treat or prevent vitamin ${{\text{B}}_{1}}$ deficiency. Thymine injection is used to treat beriberi, a condition caused by prolonged lack of vitamin ${{\text{B}}_{1}}$.
Note: The purines and pyrimidines bases are different. The purine bases include Adenine and Guanine whereas the pyrimidines include Thymine, Cytosine and Uracil. Thymine is in DNA. Thymine is replaced by uracil in RNA.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




