
The rate of decarboxylation in soda lime process for the following will be:
(a) $HC = C - COOH$
(b) $C{H_2} = CH - COOH$
(c) $C{H_3} - C{H_2} - COOH$
(A) c > b > a
(B) b > c > a
(C) a > b > c
(D) a = b = c
Answer
570k+ views
Hint: As unsaturation of molecules increases, decarboxylation becomes easier. So the rate of decarboxylation is the most in $HC = C - COOH$ followed by $C{H_2} = CH - COOH$.
Complete Solution :
The reaction in which the carboxylic acids lose carbon dioxide to form hydrocarbons when the sodium salts are heated with soda-lime is called decarboxylation. Soda lime is produced by adding sodium hydroxide solution to solid calcium oxide.
Soda lime is essentially a mixture of sodium hydroxide, calcium hydroxide and calcium oxide. It looks like white granules. It has less tendency to absorb water.
- Decarboxylation is faster in compounds with high degrees of unsaturation. Unsaturated molecules contain double bond(s), triple bond(s) and/or ring(s). Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms.
Now we have to check which compounds are highly unsaturated.
- The compound in option c does not have any double or triple bonds. Hence it is not unsaturated. So the rate of decarboxylation will be the least for it.
- We can see that both the compounds in option A and option B contain double bonds. So now we have to check which has less hydrogen. $HC = C - COOH$ has less number of hydrogen than $C{H_2} = CH - COOH$, so it is more unsaturated.
So the rate of decarboxylation is greater in $HC = C - COOH$
- Thus the order is a > b > c.
So, the correct answer is “Option C”.
Note: Decarboxylation can be alternatively defined as the process by which the carboxyl group present in an organic compound is converted to carbon dioxide. Along with this an alkane is formed as well containing 1 carbon less than the total number of carbon atoms in the reactant molecule. The reaction is given below for better understanding.
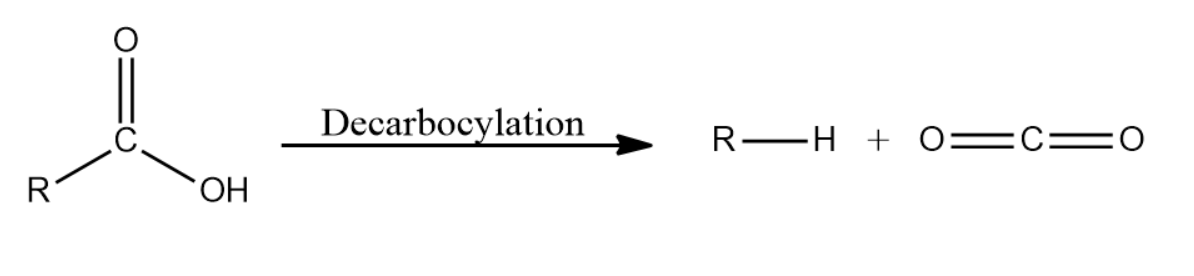
Complete Solution :
The reaction in which the carboxylic acids lose carbon dioxide to form hydrocarbons when the sodium salts are heated with soda-lime is called decarboxylation. Soda lime is produced by adding sodium hydroxide solution to solid calcium oxide.
Soda lime is essentially a mixture of sodium hydroxide, calcium hydroxide and calcium oxide. It looks like white granules. It has less tendency to absorb water.
- Decarboxylation is faster in compounds with high degrees of unsaturation. Unsaturated molecules contain double bond(s), triple bond(s) and/or ring(s). Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms.
Now we have to check which compounds are highly unsaturated.
- The compound in option c does not have any double or triple bonds. Hence it is not unsaturated. So the rate of decarboxylation will be the least for it.
- We can see that both the compounds in option A and option B contain double bonds. So now we have to check which has less hydrogen. $HC = C - COOH$ has less number of hydrogen than $C{H_2} = CH - COOH$, so it is more unsaturated.
So the rate of decarboxylation is greater in $HC = C - COOH$
- Thus the order is a > b > c.
So, the correct answer is “Option C”.
Note: Decarboxylation can be alternatively defined as the process by which the carboxyl group present in an organic compound is converted to carbon dioxide. Along with this an alkane is formed as well containing 1 carbon less than the total number of carbon atoms in the reactant molecule. The reaction is given below for better understanding.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




