
The reaction of acetaldehyde with excess of ethanol in the presence of ${{H}_{2}}S{{O}_{4}}$ gives the product
A. $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$
B. $C{{H}_{3}}CH{{(OH)}_{2}}$
C. Ketal
D. ${{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}$
Answer
546.3k+ views
Hint:Acetaldehyde could prepare acetaldehyde from the dehydrogenation of an ethanol by using a copper as catalyst in reaction. At \[260-2800{}^\circ C\] ethanol reacts over the catalyst to form acetaldehyde. Acetaldehyde was produced by hydration of the acetylene in which a mercury salt serve as catalyst before Wacker process;
\[{{C}_{2}}{{H}_{2}}+\text{ }{{H}_{2}}O\text{ }+\text{ }H{{g}_{2}}\text{ }\to \text{ }C{{H}_{3}}CHO\text{ }+\text{ }Hg\text{ }\]
Similar to this reaction ethanol reacts with acetaldehyde where ethanol is in excess amount, under presence of sulphuric acid.
Complete step-by-step answer:Acetaldehyde is also called \[MeCHO\] It is colorless organic liquid having molecular formula \[{{C}_{2}}{{H}_{4}}O\] also known \[C{{H}_{3}}CHO\] IUPAC name of Acetaldehyde is an ethanal. It has a pungent odor which is soluble in water as well as has a suffocating smell. It is non corrosive to many metals but when it has narcotic action along that can cause mucous irritation.
Ethanol or ethyl alcohol is an organic compound as well as a chemical liquid with formula \[{{C}_{2}}{{H}_{5}}OH\] It is primarily used as a solvent. In order to understand the chemical structure of an ethanol we first need to know what alkene is, Alkene are compounds that are made up of the carbon as well as hydrogen with at least one double bond among two carbon. Ethene is an alkene.
When ethanol reacts with acetaldehyde here ethanol is in excess amount under the presence of sulphuric acid it turns to produce acetal along water. Thus, acetal given by $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$
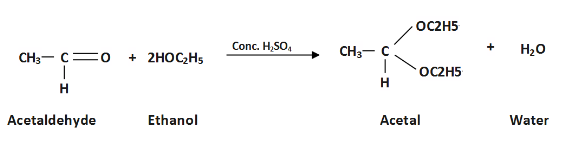
Therefore, option A $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$ is correct answer; that is reaction of acetaldehyde with excess of ethanol in presence of ${{H}_{2}}S{{O}_{4}}$ gives product $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$.
Note:Acetaldehyde is a clear liquid that burns easily. Acetaldehyde has a strong fruity odor that too high concentration could make breathing difficult. Acetaldehyde could hurt our heart as well as blood vessels. Other studies on animals show these breathing acetaldehyde can severely damage lungs as well as cause cancer. Repeated exposure to acetaldehyde in air may cause cancer in humans. When we drink alcohol our liver transforms acetaldehyde into acid. It will lead to damage to our liver.
\[{{C}_{2}}{{H}_{2}}+\text{ }{{H}_{2}}O\text{ }+\text{ }H{{g}_{2}}\text{ }\to \text{ }C{{H}_{3}}CHO\text{ }+\text{ }Hg\text{ }\]
Similar to this reaction ethanol reacts with acetaldehyde where ethanol is in excess amount, under presence of sulphuric acid.
Complete step-by-step answer:Acetaldehyde is also called \[MeCHO\] It is colorless organic liquid having molecular formula \[{{C}_{2}}{{H}_{4}}O\] also known \[C{{H}_{3}}CHO\] IUPAC name of Acetaldehyde is an ethanal. It has a pungent odor which is soluble in water as well as has a suffocating smell. It is non corrosive to many metals but when it has narcotic action along that can cause mucous irritation.
Ethanol or ethyl alcohol is an organic compound as well as a chemical liquid with formula \[{{C}_{2}}{{H}_{5}}OH\] It is primarily used as a solvent. In order to understand the chemical structure of an ethanol we first need to know what alkene is, Alkene are compounds that are made up of the carbon as well as hydrogen with at least one double bond among two carbon. Ethene is an alkene.
When ethanol reacts with acetaldehyde here ethanol is in excess amount under the presence of sulphuric acid it turns to produce acetal along water. Thus, acetal given by $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$

Therefore, option A $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$ is correct answer; that is reaction of acetaldehyde with excess of ethanol in presence of ${{H}_{2}}S{{O}_{4}}$ gives product $C{{H}_{3}}CH(O{{C}_{2}}{{H}_{5}})$.
Note:Acetaldehyde is a clear liquid that burns easily. Acetaldehyde has a strong fruity odor that too high concentration could make breathing difficult. Acetaldehyde could hurt our heart as well as blood vessels. Other studies on animals show these breathing acetaldehyde can severely damage lungs as well as cause cancer. Repeated exposure to acetaldehyde in air may cause cancer in humans. When we drink alcohol our liver transforms acetaldehyde into acid. It will lead to damage to our liver.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




