
The reaction of aniline with chloroform under alkaline conditions lead to the formation of:
A) Phenyl cyanide
B) Phenyl isonitrile
C) Phenyl cyanate
D) Phenyl isocyanate
Answer
573.9k+ views
Hint: Aliphatic and the aromatic primary amines when warmed with chloroform with the chloroform and an alcoholic solution of $\text{ KOH }$ , form isocyanides or carbylamines which have a very unpleasant or foul smell. The general reaction of the amine is given as follows,
$\begin{matrix}
\text{R}-\text{N}{{\text{H}}_{\text{2}}} & \text{+} & \text{CHC}{{\text{l}}_{\text{3}}} & \text{+} & \text{3KOH(alc}\text{.)} & \xrightarrow{\text{warm}} & \text{R}-\text{NC} & \text{+} & \text{RNC} & \text{+} & \text{3}{{\text{H}}_{\text{2}}}\text{O} \\
{{1}^{\text{o}}}\text{amine} & {} & {} & {} & {} & {} & \text{Alkyl isocyanide} & {} & {} & {} &
{} \\
\end{matrix}\text{ }$
Complete step by step answer:
Aniline is an aromatic amine. its general structural formula is $\text{ Ar}-\text{N}{{\text{H}}_{\text{2}}}\text{ }$. It contains the amine $\text{ }-\text{N}{{\text{H}}_{\text{2}}}\text{ }$ group. When aniline is warmed with the chloroform in the alcoholic alkaline solution, it generates an organic product which has a distinct bad or foul sell .this compound is an isonitrile .the reaction of aniline is as shown below,
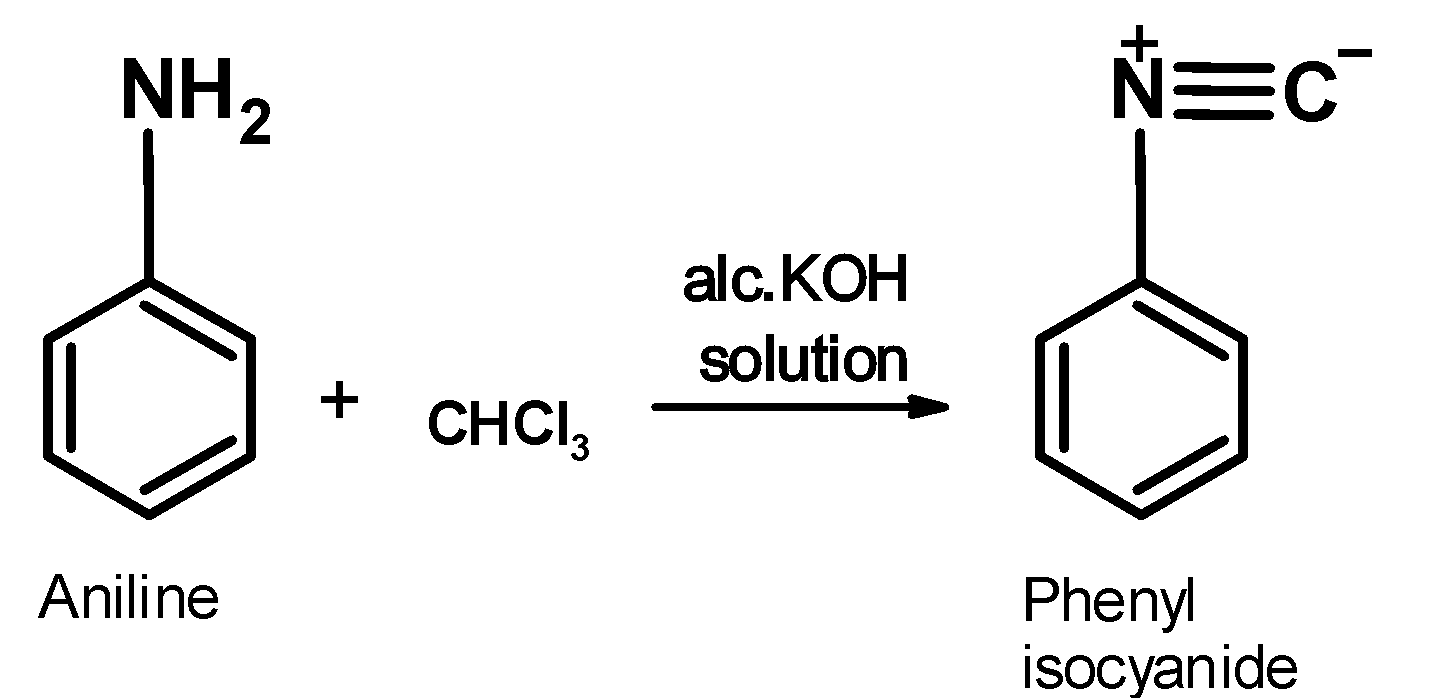
The mechanist pathways for the carbylamine test is as shown below,
In step 1, the dichlorocarbene derived from the chloroform is attacked by the lone pair of electrons on the nitrogen atom of the amine. This generates an intermediate by the dehydrohalogenation of the chloroform.
In the next step, the base i.e. potassium hydroxide in the alcoholic solution abstracts two protons from the intermediates in the successive step. This generates an isocyanides product. The entire mechanistic pathway for the reaction is as shown below,
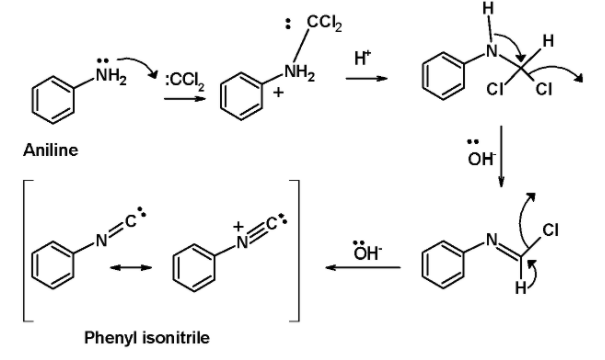
Aromatic amine ‘aniline’ reacts with the chloroform in an alkaline solution leading to the formation of phenyl isocyanides or phenyl isonitrile.
Hence, (B) is the correct option.
Note: Note that, the secondary or the tertiary amines do not give the carbylamine test. This test is used to distinguish between the primary amines from the secondary and the tertiary amines. Isocyanides are also known as the isonitrile same as we call cyanide and nitrile. Remember that here the position of attachment has a significant role in determining the name. It attaches through N thus known as isonitrile.
$\begin{matrix}
\text{R}-\text{N}{{\text{H}}_{\text{2}}} & \text{+} & \text{CHC}{{\text{l}}_{\text{3}}} & \text{+} & \text{3KOH(alc}\text{.)} & \xrightarrow{\text{warm}} & \text{R}-\text{NC} & \text{+} & \text{RNC} & \text{+} & \text{3}{{\text{H}}_{\text{2}}}\text{O} \\
{{1}^{\text{o}}}\text{amine} & {} & {} & {} & {} & {} & \text{Alkyl isocyanide} & {} & {} & {} &
{} \\
\end{matrix}\text{ }$
Complete step by step answer:
Aniline is an aromatic amine. its general structural formula is $\text{ Ar}-\text{N}{{\text{H}}_{\text{2}}}\text{ }$. It contains the amine $\text{ }-\text{N}{{\text{H}}_{\text{2}}}\text{ }$ group. When aniline is warmed with the chloroform in the alcoholic alkaline solution, it generates an organic product which has a distinct bad or foul sell .this compound is an isonitrile .the reaction of aniline is as shown below,

The mechanist pathways for the carbylamine test is as shown below,
In step 1, the dichlorocarbene derived from the chloroform is attacked by the lone pair of electrons on the nitrogen atom of the amine. This generates an intermediate by the dehydrohalogenation of the chloroform.
In the next step, the base i.e. potassium hydroxide in the alcoholic solution abstracts two protons from the intermediates in the successive step. This generates an isocyanides product. The entire mechanistic pathway for the reaction is as shown below,

Aromatic amine ‘aniline’ reacts with the chloroform in an alkaline solution leading to the formation of phenyl isocyanides or phenyl isonitrile.
Hence, (B) is the correct option.
Note: Note that, the secondary or the tertiary amines do not give the carbylamine test. This test is used to distinguish between the primary amines from the secondary and the tertiary amines. Isocyanides are also known as the isonitrile same as we call cyanide and nitrile. Remember that here the position of attachment has a significant role in determining the name. It attaches through N thus known as isonitrile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




