
The reaction of ${{C}_{6}}{{H}_{5}}CH=CHC{{H}_{3}}$ with $HBr$ produces?
A. ${{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Br$
B.
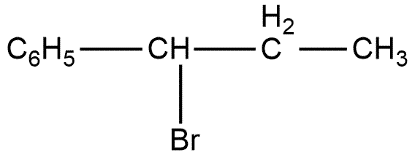
C.
D.
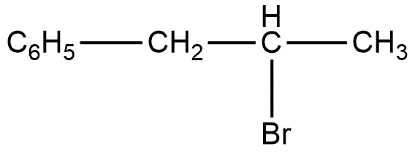


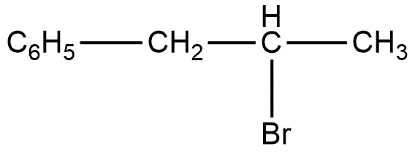
Answer
516.6k+ views
Hint: Chemical reactions are those reactions in which reactants get converted into product under some conditions like at fixed temperature and pressure and some reactions proceed with the help of catalysts which increase their rate of reaction.
Complete answer:
The reaction of ${{C}_{6}}{{H}_{5}}CH=CHC{{H}_{3}}$ with $HBr$ can be defined on the basis of property called resonance where resonance describes the delocalized electrons within the certain molecules where one single lewis structure does not express the bonds. An ion or molecule with these delocalized electrons can be represented by a number of structures which are called resonance structures.
Resonance or Mesomerism describes the delocalized electrons within the certain molecules where one single lewis structure does not express the bonds. An ion or molecule with these delocalized electrons can be represented by a number of structures which are called resonance structures.
The reaction ${{C}_{6}}{{H}_{5}}CH=CHC{{H}_{3}}$ with $HBr$ produces product given in option C. This can be shown as follows:
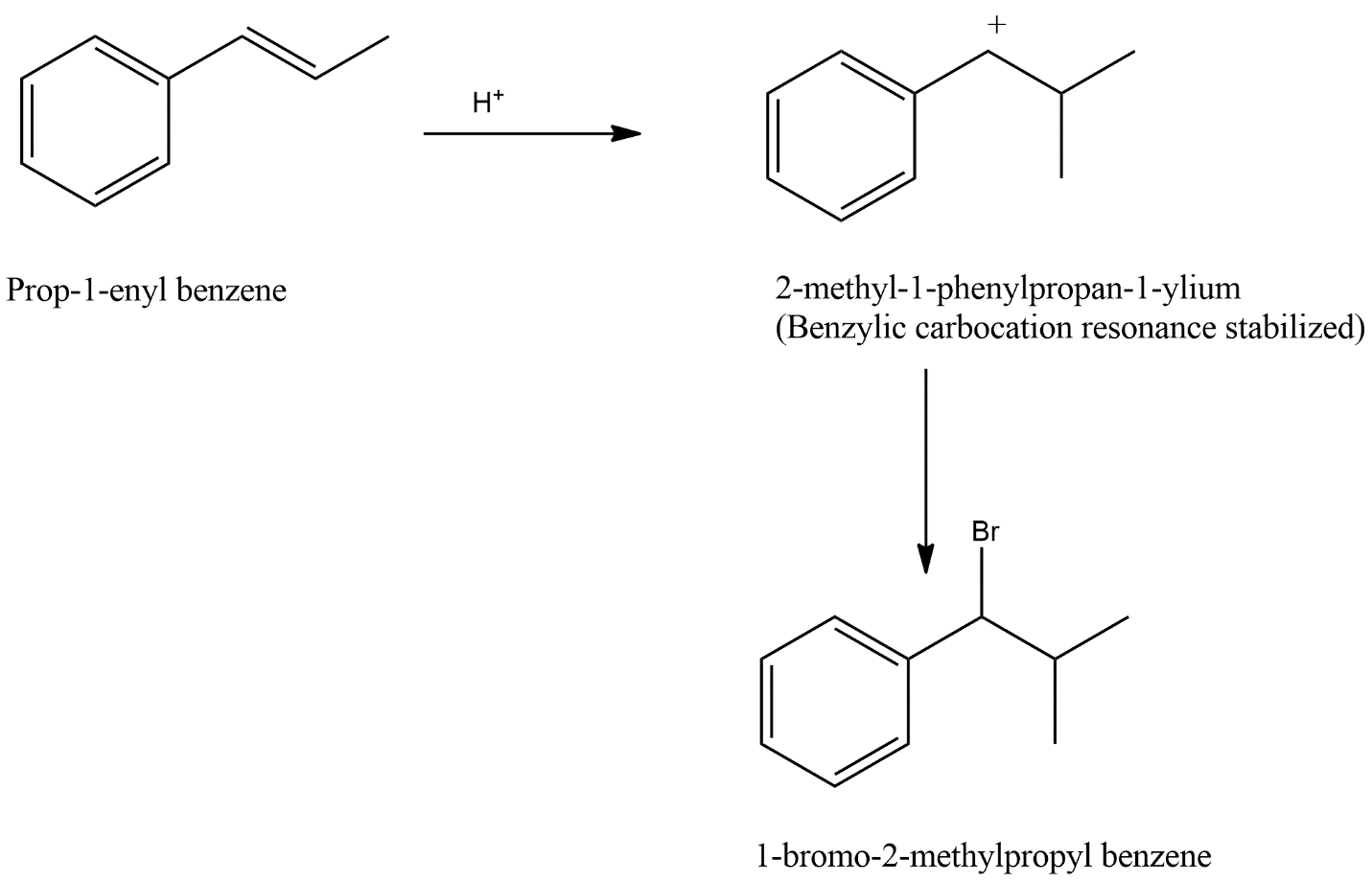
Hence before forming the main product an intermediate product is formed which is resonance stabilized and have carbocation like character where carbocation are those substances which have in which a carbon atom has a positive charge also we can consider that carbocation is made up of two words carbo and cation where carbon is for carbon and cation is positively charged ions.
Hence from the above reaction we can say that option C is the correct answer.
Note:
Stability of the carbocation is generally dependent upon the resonance, inductive or hyperconjugation effect and electronegativity value of the compound which can be explained as stability of the carbocation increases with the increase in number of resonating structures.
Complete answer:
The reaction of ${{C}_{6}}{{H}_{5}}CH=CHC{{H}_{3}}$ with $HBr$ can be defined on the basis of property called resonance where resonance describes the delocalized electrons within the certain molecules where one single lewis structure does not express the bonds. An ion or molecule with these delocalized electrons can be represented by a number of structures which are called resonance structures.
Resonance or Mesomerism describes the delocalized electrons within the certain molecules where one single lewis structure does not express the bonds. An ion or molecule with these delocalized electrons can be represented by a number of structures which are called resonance structures.
The reaction ${{C}_{6}}{{H}_{5}}CH=CHC{{H}_{3}}$ with $HBr$ produces product given in option C. This can be shown as follows:

Hence before forming the main product an intermediate product is formed which is resonance stabilized and have carbocation like character where carbocation are those substances which have in which a carbon atom has a positive charge also we can consider that carbocation is made up of two words carbo and cation where carbon is for carbon and cation is positively charged ions.
Hence from the above reaction we can say that option C is the correct answer.
Note:
Stability of the carbocation is generally dependent upon the resonance, inductive or hyperconjugation effect and electronegativity value of the compound which can be explained as stability of the carbocation increases with the increase in number of resonating structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




