
The reagent needed for converting is:
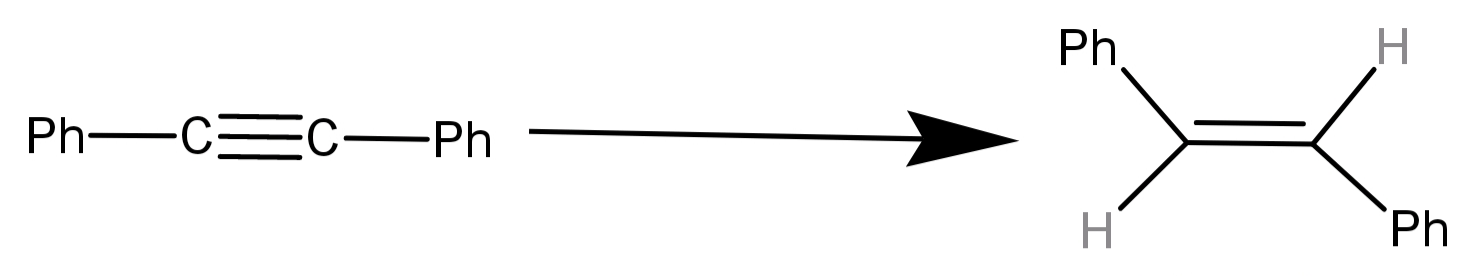
(A) Catalytic hydrogenation
(B) ${H_2}$ / Lindlar’s catalyst
(C) $LiAl{H_4}$
(D) $Li/N{H_3}$

Answer
586.2k+ views
Hint: Here an alkyne has been converted into a trans alkene. This reagent is a reducing agent which undergoes addition of hydrogen across the triple bond by addition of solvated electrons and hence one of its components will act as a solvent. This reagent is also known as Birch’s reagent.
Complete step by step answer:
-We can see in the reaction that the alkyne has been converted into alkene and to be specific the alkene is a trans alkene. This is also said that there has been addition of an hydrogen atom at both sides of the triple bond to convert it into a double bond. Hence this is an addition reaction.
-Hence we will now talk about addition reactions.
We should know that unsaturated hydrocarbons can participate in a number of different addition reactions across their double or triple bonds. Also the addition reactions include catalytic hydrogenation (addition of hydrogen), halogenation (reaction with halogen), and hydrohalogenation (reaction with H-X, where X is a halogen).
-We will now check all the options and see which one of them can cause the given reaction.
-For (A) Catalytic hydrogenation: It is the reduction of alkynes to alkanes or alkenes to alkanes in the presence of a metal catalyst such as platinum, palladium, nickel, or rhodium. This leads to the addition of hydrogen atoms across the double or triple bonds. But the major thing about catalytic hydrogenation is that this reaction does not stop an alkene stage and continues further to form an alkane.
Hence the above reaction cannot be done via catalytic hydrogenation because its end product is an alkene.
-For (B) ${H_2}$ / Lindlar’s catalyst: The Lindlar’s catalyst is a catalyst made up of palladium (Pd) deposited on calcium carbonate ($CaC{O_3}$) or barium sulphate ($BaS{O_4}$) poisoned with traces of lead (Pb) and quinoline. It is used for the conversion of alkynes to alkenes, but the alkene product is always a cis product.
The given reaction cannot be done via Lindlar’s catalyst also since the product form in the question is a trans product.
-For (C) $LiAl{H_4}$: It is used as a reducing agent for esters, carboxylic acids and amides. But it does not affect the double or triple bonds.
Hence $LiAl{H_4}$ also cannot be used for the given reaction.
-For (D) $Li/N{H_3}$: This is known as Birch’s reagent and is used to convert alkynes to trans alkenes. Here the hydrogen atoms are added across the triple bond via addition of solvated electrons from ammonia ($N{H_3}$).
Hence $Li/N{H_3}$ can be used for the given reaction.
So, the correct answer is “Option D”.
Note: Do not get confused between option (B) ${H_2}$ / Lindlar’s catalyst and option (D) $Li/N{H_3}$ since both convert alkynes to alkenes. But the difference is that Lindlar’s catalyst will convert them into cis products while $Li/N{H_3}$ (Birch’s reagent) will convert them into trans products. Also the Birch’s reaction is an organic redox reaction where $N{H_3}$ (ammonia) acts as a solvent (tetrahydrofuran or THF can also be used as a solvent). Alkali metals like Li give a blue coloured solution when dissolved in liquid ammonia and hence this reagent is blue in colour.
Complete step by step answer:
-We can see in the reaction that the alkyne has been converted into alkene and to be specific the alkene is a trans alkene. This is also said that there has been addition of an hydrogen atom at both sides of the triple bond to convert it into a double bond. Hence this is an addition reaction.
-Hence we will now talk about addition reactions.
We should know that unsaturated hydrocarbons can participate in a number of different addition reactions across their double or triple bonds. Also the addition reactions include catalytic hydrogenation (addition of hydrogen), halogenation (reaction with halogen), and hydrohalogenation (reaction with H-X, where X is a halogen).
-We will now check all the options and see which one of them can cause the given reaction.
-For (A) Catalytic hydrogenation: It is the reduction of alkynes to alkanes or alkenes to alkanes in the presence of a metal catalyst such as platinum, palladium, nickel, or rhodium. This leads to the addition of hydrogen atoms across the double or triple bonds. But the major thing about catalytic hydrogenation is that this reaction does not stop an alkene stage and continues further to form an alkane.
Hence the above reaction cannot be done via catalytic hydrogenation because its end product is an alkene.
-For (B) ${H_2}$ / Lindlar’s catalyst: The Lindlar’s catalyst is a catalyst made up of palladium (Pd) deposited on calcium carbonate ($CaC{O_3}$) or barium sulphate ($BaS{O_4}$) poisoned with traces of lead (Pb) and quinoline. It is used for the conversion of alkynes to alkenes, but the alkene product is always a cis product.
The given reaction cannot be done via Lindlar’s catalyst also since the product form in the question is a trans product.
-For (C) $LiAl{H_4}$: It is used as a reducing agent for esters, carboxylic acids and amides. But it does not affect the double or triple bonds.
Hence $LiAl{H_4}$ also cannot be used for the given reaction.
-For (D) $Li/N{H_3}$: This is known as Birch’s reagent and is used to convert alkynes to trans alkenes. Here the hydrogen atoms are added across the triple bond via addition of solvated electrons from ammonia ($N{H_3}$).
Hence $Li/N{H_3}$ can be used for the given reaction.
So, the correct answer is “Option D”.
Note: Do not get confused between option (B) ${H_2}$ / Lindlar’s catalyst and option (D) $Li/N{H_3}$ since both convert alkynes to alkenes. But the difference is that Lindlar’s catalyst will convert them into cis products while $Li/N{H_3}$ (Birch’s reagent) will convert them into trans products. Also the Birch’s reaction is an organic redox reaction where $N{H_3}$ (ammonia) acts as a solvent (tetrahydrofuran or THF can also be used as a solvent). Alkali metals like Li give a blue coloured solution when dissolved in liquid ammonia and hence this reagent is blue in colour.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




