
: The red curve in the figure below shows the variation in potential energy of the system (vertical axis) as a function of the distance (horizontal axis) between two atoms as they move together to form a bond. The atoms are approaching from infinite separation (far right) toward each other. The origin is taken to be \[\left( {0,0} \right)\] .
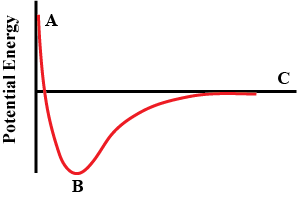
Which of the following best accounts for the change in the potential energy of the system going from Point B to Point A?
A.The bond is becoming stronger as the atoms approaches each other
B.As the molecules gets closer , they slow down, converting kinetic energy to potential energy
C.As the atoms gets closer, the attraction of the nucleus of each atom for the electron(s) of other atom, gets stronger and stronger
D.As the atoms gets closer, their nuclei begin to repel one another

Answer
573.3k+ views
Hint: Formation of chemical bond is an exothermic process; more the energy is decreased, stronger will be the bond. If the distance between the molecules reduces than a certain limit then they tend to repel each other.
Complete answer:
A molecule will only be formed if it is more stable and also has a lower energy than the individual atoms. Thus a chemical bond is formed between two or more atoms which act as a force to hold them together as a stable molecule. This process is accompanied by a decrease in energy. Strength of chemical bonds is directly proportional to the amount of energy decreased. The cause for the bond formation could be the tendency to acquire minimum energy and the tendency to acquire noble gas configuration.
As given in the question, the potential energy existing between two atoms which are forming chemical bonds is the function of distance between them. As we can see that when the distance between atoms is infinite or when atoms are placed at greater distance the potential energy existing between them is almost zero which means no chemical bond exists between them. Now moving toward origin from infinity or from point C to B according to the given graph, we can see potential energy existing between atoms is getting negative which suggests that energy is decreasing between them or the chemical bond is getting stronger. Strength of bond is directly proportional to amount of energy released. From C to B, when two atoms approach each other, the nucleus of one atom attracts the electron of another atom. The decrease in energy is only up to certain limit (point B) At this point the chemical bond between them is the strongest. On further moving towards origin or we can say for further decrease in separation between atoms, the potential energy starts increasing ( from point B to A), thereby making chemical bonds between atoms weaker due to increased amount of energy. This is due to the greater repulsion between the nuclei of approaching atoms. The nuclei of atoms have the same charge that is positive and positive charge repels each other.
Thus, the correct option is D.
Note:
The tendency of an atom to achieve eight electrons in their outermost shell is known as Lewis octet rule. According to the Sidgwick concept, a central atom in a molecule can have more than 8 electrons as the maximum covalency depends on the period to which it belongs.
Complete answer:
A molecule will only be formed if it is more stable and also has a lower energy than the individual atoms. Thus a chemical bond is formed between two or more atoms which act as a force to hold them together as a stable molecule. This process is accompanied by a decrease in energy. Strength of chemical bonds is directly proportional to the amount of energy decreased. The cause for the bond formation could be the tendency to acquire minimum energy and the tendency to acquire noble gas configuration.
As given in the question, the potential energy existing between two atoms which are forming chemical bonds is the function of distance between them. As we can see that when the distance between atoms is infinite or when atoms are placed at greater distance the potential energy existing between them is almost zero which means no chemical bond exists between them. Now moving toward origin from infinity or from point C to B according to the given graph, we can see potential energy existing between atoms is getting negative which suggests that energy is decreasing between them or the chemical bond is getting stronger. Strength of bond is directly proportional to amount of energy released. From C to B, when two atoms approach each other, the nucleus of one atom attracts the electron of another atom. The decrease in energy is only up to certain limit (point B) At this point the chemical bond between them is the strongest. On further moving towards origin or we can say for further decrease in separation between atoms, the potential energy starts increasing ( from point B to A), thereby making chemical bonds between atoms weaker due to increased amount of energy. This is due to the greater repulsion between the nuclei of approaching atoms. The nuclei of atoms have the same charge that is positive and positive charge repels each other.
Thus, the correct option is D.
Note:
The tendency of an atom to achieve eight electrons in their outermost shell is known as Lewis octet rule. According to the Sidgwick concept, a central atom in a molecule can have more than 8 electrons as the maximum covalency depends on the period to which it belongs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




